Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons
- PMID: 33882218
- PMCID: PMC8117969
- DOI: 10.1056/NEJMoa2104983
Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons
Erratum in
-
Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons.N Engl J Med. 2021 Oct 14;385(16):1536. doi: 10.1056/NEJMx210016. Epub 2021 Sep 8. N Engl J Med. 2021. PMID: 34496171 No abstract available.
Abstract
Background: Many pregnant persons in the United States are receiving messenger RNA (mRNA) coronavirus disease 2019 (Covid-19) vaccines, but data are limited on their safety in pregnancy.
Methods: From December 14, 2020, to February 28, 2021, we used data from the "v-safe after vaccination health checker" surveillance system, the v-safe pregnancy registry, and the Vaccine Adverse Event Reporting System (VAERS) to characterize the initial safety of mRNA Covid-19 vaccines in pregnant persons.
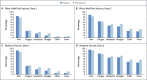
Results: A total of 35,691 v-safe participants 16 to 54 years of age identified as pregnant. Injection-site pain was reported more frequently among pregnant persons than among nonpregnant women, whereas headache, myalgia, chills, and fever were reported less frequently. Among 3958 participants enrolled in the v-safe pregnancy registry, 827 had a completed pregnancy, of which 115 (13.9%) resulted in a pregnancy loss and 712 (86.1%) resulted in a live birth (mostly among participants with vaccination in the third trimester). Adverse neonatal outcomes included preterm birth (in 9.4%) and small size for gestational age (in 3.2%); no neonatal deaths were reported. Although not directly comparable, calculated proportions of adverse pregnancy and neonatal outcomes in persons vaccinated against Covid-19 who had a completed pregnancy were similar to incidences reported in studies involving pregnant women that were conducted before the Covid-19 pandemic. Among 221 pregnancy-related adverse events reported to the VAERS, the most frequently reported event was spontaneous abortion (46 cases).
Conclusions: Preliminary findings did not show obvious safety signals among pregnant persons who received mRNA Covid-19 vaccines. However, more longitudinal follow-up, including follow-up of large numbers of women vaccinated earlier in pregnancy, is necessary to inform maternal, pregnancy, and infant outcomes.
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
Safety and efficacy of COVID-19 vaccines in pregnant women with rheumatic diseases: an immunologic perspective.Rheumatol Int. 2021 Aug;41(8):1545-1547. doi: 10.1007/s00296-021-04918-z. Epub 2021 Jun 10. Rheumatol Int. 2021. PMID: 34110465 Free PMC article. No abstract available.
-
mRNA Covid-19 Vaccines in Pregnant Women.N Engl J Med. 2021 Jun 17;384(24):2342-2343. doi: 10.1056/NEJMe2107070. N Engl J Med. 2021. PMID: 34133864 Free PMC article. No abstract available.
-
COVID-19 vaccines and pregnancy: What do we know?Therapie. 2021 Jul-Aug;76(4):373-374. doi: 10.1016/j.therap.2021.05.011. Epub 2021 Jun 1. Therapie. 2021. PMID: 34238585 Free PMC article. No abstract available.
-
On Preliminary Findings of mRNA Covid-19 Vaccine Safety in Pregnant Persons.N Engl J Med. 2021 Oct 14;385(16):1535-1536. doi: 10.1056/NEJMc2113516. Epub 2021 Sep 8. N Engl J Med. 2021. PMID: 34496197 No abstract available.
References
-
- Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). 2021. (https://www.fda.gov/media/144413/download).
-
- Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). 2021. (https://www.fda.gov/media/144637/download).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
