Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19
- PMID: 33882225
- PMCID: PMC8220996
- DOI: 10.1056/NEJMoa2101544
Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19
Abstract
Background: The Ad26.COV2.S vaccine is a recombinant, replication-incompetent human adenovirus type 26 vector encoding full-length severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein in a prefusion-stabilized conformation.
Methods: In an international, randomized, double-blind, placebo-controlled, phase 3 trial, we randomly assigned adult participants in a 1:1 ratio to receive a single dose of Ad26.COV2.S (5×1010 viral particles) or placebo. The primary end points were vaccine efficacy against moderate to severe-critical coronavirus disease 2019 (Covid-19) with an onset at least 14 days and at least 28 days after administration among participants in the per-protocol population who had tested negative for SARS-CoV-2. Safety was also assessed.
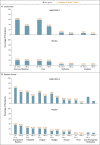
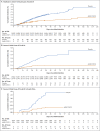
Results: The per-protocol population included 19,630 SARS-CoV-2-negative participants who received Ad26.COV2.S and 19,691 who received placebo. Ad26.COV2.S protected against moderate to severe-critical Covid-19 with onset at least 14 days after administration (116 cases in the vaccine group vs. 348 in the placebo group; efficacy, 66.9%; adjusted 95% confidence interval [CI], 59.0 to 73.4) and at least 28 days after administration (66 vs. 193 cases; efficacy, 66.1%; adjusted 95% CI, 55.0 to 74.8). Vaccine efficacy was higher against severe-critical Covid-19 (76.7% [adjusted 95% CI, 54.6 to 89.1] for onset at ≥14 days and 85.4% [adjusted 95% CI, 54.2 to 96.9] for onset at ≥28 days). Despite 86 of 91 cases (94.5%) in South Africa with sequenced virus having the 20H/501Y.V2 variant, vaccine efficacy was 52.0% and 64.0% against moderate to severe-critical Covid-19 with onset at least 14 days and at least 28 days after administration, respectively, and efficacy against severe-critical Covid-19 was 73.1% and 81.7%, respectively. Reactogenicity was higher with Ad26.COV2.S than with placebo but was generally mild to moderate and transient. The incidence of serious adverse events was balanced between the two groups. Three deaths occurred in the vaccine group (none were Covid-19-related), and 16 in the placebo group (5 were Covid-19-related).
Conclusions: A single dose of Ad26.COV2.S protected against symptomatic Covid-19 and asymptomatic SARS-CoV-2 infection and was effective against severe-critical disease, including hospitalization and death. Safety appeared to be similar to that in other phase 3 trials of Covid-19 vaccines. (Funded by Janssen Research and Development and others; ENSEMBLE ClinicalTrials.gov number, NCT04505722.).
Copyright © 2021 Massachusetts Medical Society.
Figures
Comment in
-
Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19.N Engl J Med. 2021 Jul 15;385(3):288. doi: 10.1056/NEJMc2107809. Epub 2021 Jun 9. N Engl J Med. 2021. PMID: 34107178 No abstract available.
-
The single-dose J&J vaccine had 67% efficacy against moderate to severe-critical COVID-19 at ≥14 d.Ann Intern Med. 2021 Jul;174(7):JC75. doi: 10.7326/ACPJ202107200-075. Epub 2021 Jul 6. Ann Intern Med. 2021. PMID: 34224270
References
-
- Johns Hopkins University Coronavirus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2021. (https://coronavirus.jhu.edu/map.html).
-
- Tegally H, Wilkinson E, Giovanetti M, et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. December 22, 2020. (https://www.medrxiv.org/content/10.1101/2020.12.21.20248640v1). preprint.
-
- Public Health England. Investigation of novel SARS-CoV-2 variant: variant of concern 202012/01: technical briefing 3. 2021. (https://assets.publishing.service.gov.uk/government/uploads/system/uploa...).
-
- Krammer F. SARS-CoV-2 vaccines in development. Nature 2020;586:516-527. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 AI068636/AI/NIAID NIH HHS/United States
- UM1 AI069476/AI/NIAID NIH HHS/United States
- U01 AI068619/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- UM1 AI069469/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- UM1 AI068619/AI/NIAID NIH HHS/United States
- U01 AI068635/AI/NIAID NIH HHS/United States
- U01 AI068614/AI/NIAID NIH HHS/United States
- U01 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- U01 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- UM1 AI148685/AI/NIAID NIH HHS/United States
- S10 AI174104/AI/NIAID NIH HHS/United States
- P30 AI045008/AI/NIAID NIH HHS/United States
- UM1 AI148452/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
