Bufadienolides from the Eggs of the Toad Bufo bufo gargarizans and Their Antimelanoma Activities
- PMID: 33882233
- PMCID: PMC9042390
- DOI: 10.1021/acs.jnatprod.0c00840
Bufadienolides from the Eggs of the Toad Bufo bufo gargarizans and Their Antimelanoma Activities
Abstract
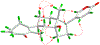
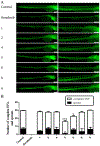
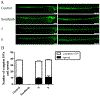
Toads produce potent toxins, named bufadienolides, to defend against their predators. Pharmacological research has revealed that bufadienolides are potential anticancer drugs. In this research, we reported nine bufadienolides from the eggs of the toad Bufo bufo gargarizans, including two new compounds (1 and 3). The chemical structures of 1 and 3, as well as of one previously reported semisynthesized compound (2), were elucidated on the basis of extensive spectroscopic data interpretation, chemical methods, and X-ray diffraction analysis. Compound 1 is an unusual 19-norbufadienolide with rearranged A/B rings. A biological test revealed that compounds 2 and 4-8 showed potent cytotoxic activities toward human melanoma cell line SK-MEL-1 with IC50 values less than 1.0 μM. A preliminary mechanism investigation revealed that the most potent compound, 8, could induce apoptosis via PARP cleavage, while 5 and 6 significantly suppressed angiogenesis in zebrafish. Furthermore, an in vivo biological study showed that 5, 6, and 8 inhibit SK-MEL-1 cell growth significantly.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

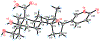



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

