Performance characteristics of five antigen-detecting rapid diagnostic test (Ag-RDT) for SARS-CoV-2 asymptomatic infection: a head-to-head benchmark comparison
- PMID: 33882299
- PMCID: PMC8053403
- DOI: 10.1016/j.jinf.2021.04.009
Performance characteristics of five antigen-detecting rapid diagnostic test (Ag-RDT) for SARS-CoV-2 asymptomatic infection: a head-to-head benchmark comparison
Abstract
Background: Mass testing for early identification and isolation of infectious COVID-19 individuals is efficacious for reducing disease spread. Antigen-detecting rapid diagnostic tests (Ag-RDT) may be suitable for testing strategies; however, benchmark comparisons are scarce.
Methods: We used 286 nasopharyngeal specimens from unexposed asymptomatic individuals collected between December 2020 and January 2021 to assess five Ag-RDTs marketed by Abbott, Siemens, Roche Diagnostics, Lepu Medical, and Surescreen.
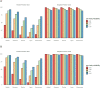
Results: For the overall sample, the performance parameters of Ag-RDTs were as follows: Abbott assay, sensitivity 38.6% (95%CI 29.1-48.8) and specificity 99.5% (97-100%); Siemens, sensitivity 51.5% (41.3-61.6) and specificity 98.4% (95.3-99.6); Roche, sensitivity 43.6% (33.7-53.8) and specificity 96.2% (92.4-98.5); Lepu, sensitivity 45.5% (35.6-55.8) and specificity 89.2% (83.8-93.3%); Surescreen, sensitivity 28.8% (20.2-38.6) and specificity 97.8% (94.5-99.4%). For specimens with cycle threshold (Ct) <30 in RT-qPCR, all Ag-RDT achieved a sensitivity ≥70%. The modelled negative- and positive-predictive value for 1% prevalence were >99% and <50%, respectively.
Conclusions: When screening unexposed asymptomatic individuals, two Ag-RDTs achieved sensitivity ≥80% for specimens with Ct<30 and specificity ≥96%. The estimated negative predictive value suggests the suitability of Ag-RDTs for mass screenings of SARS-CoV-2 infection in the general population.
Keywords: Antigen-detecting rapid diagnostic test; Head-to-head comparison; Mass screening; SARS-CoV-2.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest We declare no conflicts of interest.
Figures



References
-
- Pavelka M, Van-Zandvoort K, Abbott S, Sherratt K, Majdan M, Jarčuška P, et al. The effectiveness of population-wide, rapid antigen test based screening in reducing SARS-CoV-2 infection prevalence in Slovakia. medRxiv 2020.
-
- World Health Organization (WHO). Diagnostic testing for SARS-CoV-2. Available at: https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2. Accessed 6 October 2020.
-
- Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA – J Am Med Assoc. 2020;323:2249–2251. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

