Chronic loss of inhibition in piriform cortex following brief, daily optogenetic stimulation
- PMID: 33882304
- PMCID: PMC8102022
- DOI: 10.1016/j.celrep.2021.109001
Chronic loss of inhibition in piriform cortex following brief, daily optogenetic stimulation
Abstract
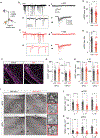
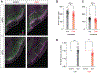
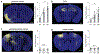
It is well established that seizures beget seizures, yet the cellular processes that underlie progressive epileptogenesis remain unclear. Here, we use optogenetics to briefly activate targeted populations of mouse piriform cortex (PCx) principal neurons in vivo. After just 3 or 4 days of stimulation, previously subconvulsive stimuli trigger massive, generalized seizures. Highly recurrent allocortices are especially prone to "optokindling." Optokindling upsets the balance of recurrent excitation and feedback inhibition. To understand how this balance is disrupted, we then selectively reactivate the same neurons in vitro. Surprisingly, we find no evidence of heterosynaptic potentiation; instead, we observe a marked, pathway-specific decrease in feedback inhibition. We find no loss of inhibitory interneurons; rather, decreased GABA synthesis in feedback inhibitory neurons appears to underlie weakened inhibition. Optokindling will allow precise identification of the molecular processes by which brain activity patterns can progressively and pathologically disrupt the balance of cortical excitation and inhibition.
Keywords: GABA depletion; channelrhodopsin-2; epilepsy; kindling; olfactory cortex; optogenetics; optokindling; piriform cortex; seizures.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

