Minimal specifications for non-human primate MRI: Challenges in standardizing and harmonizing data collection
- PMID: 33882349
- PMCID: PMC8594288
- DOI: 10.1016/j.neuroimage.2021.118082
Minimal specifications for non-human primate MRI: Challenges in standardizing and harmonizing data collection
Abstract
Recent methodological advances in MRI have enabled substantial growth in neuroimaging studies of non-human primates (NHPs), while open data-sharing through the PRIME-DE initiative has increased the availability of NHP MRI data and the need for robust multi-subject multi-center analyses. Streamlined acquisition and analysis protocols would accelerate and improve these efforts. However, consensus on minimal standards for data acquisition protocols and analysis pipelines for NHP imaging remains to be established, particularly for multi-center studies. Here, we draw parallels between NHP and human neuroimaging and provide minimal guidelines for harmonizing and standardizing data acquisition. We advocate robust translation of widely used open-access toolkits that are well established for analyzing human data. We also encourage the use of validated, automated pre-processing tools for analyzing NHP data sets. These guidelines aim to refine methodological and analytical strategies for small and large-scale NHP neuroimaging data. This will improve reproducibility of results, and accelerate the convergence between NHP and human neuroimaging strategies which will ultimately benefit fundamental and translational brain science.
Keywords: MRI; Multi-site; Non-human primate; PRIME-DE; Standardization.
Copyright © 2021. Published by Elsevier Inc.
Figures
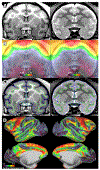

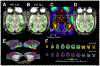
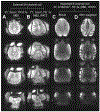





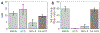
References
-
- Autio JA, Glasser MF, Ose T, Donahue CJ, Bastiani M, Ohno M, Kawabata Y, Urushibata Y, Murata K, Nishigori K, Yamaguchi M, Hori Y, Yoshida A, Go Y, Coalson TS, Jbabdi S, Sotiropoulos SN, Kennedy H, Smith S, Van Essen DC, Hayashi T, 2020. Towards HCP-Style macaque connectomes: 24-Channel 3T multi-array coil, MRI sequences and preprocessing. NeuroImage 215, 116800. doi: 10.1016/j.neuroimage.2020.116800. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- P41 EB027061/EB/NIBIB NIH HHS/United States
- FRN-148453/CIHR/Canada
- R01 MH101555/MH/NIMH NIH HHS/United States
- P30 DA048742/DA/NIDA NIH HHS/United States
- R01 MH096773/MH/NIMH NIH HHS/United States
- U54 MH091657/MH/NIMH NIH HHS/United States
- P50 MH109429/MH/NIMH NIH HHS/United States
- U01 DA041148/DA/NIDA NIH HHS/United States
- RF1 MH117040/MH/NIMH NIH HHS/United States
- R01 MH115357/MH/NIMH NIH HHS/United States
- R01 MH111439/MH/NIMH NIH HHS/United States
- P50 NS098573/NS/NINDS NIH HHS/United States
- RF1 MH116978/MH/NIMH NIH HHS/United States
- R01 MH060974/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

