A multi-centre, randomized, controlled trial on coaching and telemonitoring in patients with cystic fibrosis: conneCT CF
- PMID: 33882893
- PMCID: PMC8058751
- DOI: 10.1186/s12890-021-01500-y
A multi-centre, randomized, controlled trial on coaching and telemonitoring in patients with cystic fibrosis: conneCT CF
Abstract
Background: The extend of lung disease remains the most important prognostic factor for survival in patients with cystic fibrosis (CF), and lack of adherence is the main reason for treatment failure. Early detection of deterioration in lung function and optimising adherence are therefore crucial in CF care. We implement a randomized controlled trial to evaluate efficacy of telemonitoring of adherence, lung function, and health condition in combination with behavior change interventions using innovative digital technologies.
Methods: This is a multi-centre, randomized, controlled, non-blinded trial aiming to include 402 patients ≥ 12 years-of-age with CF. A standard-of-care arm is compared to an arm receiving objective, continuous monitoring of adherence to inhalation therapies, weekly home spirometry using electronic devices with data transmission to patients and caring physicians combined with video-conferencing, a self-management app and professional telephone coaching. The duration of the intervention phase is 18 months. The primary endpoint is time to the first protocol-defined pulmonary exacerbation. Secondary outcome measures include number of and time between pulmonary exacerbations, adherence to inhalation therapy, changes in forced expiratory volume in 1 s from baseline, number of hospital admissions, and changes in health-related quality of life. CF-associated medical treatment and care, and health care related costs will be assessed by explorative analysis in both arms.
Discussion: This study offers the opportunity to evaluate the effect of adherence interventions using telemedicine capable devices on adherence and lung health, possibly paving the way for implementation of telemedicine in routine care for patients with CF.
Trial registration: This study has been registered with the German Clinical Trials Register (Identifier: DRKS00024642, date of registration 01 Mar 2021, URL: https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00024642 ).
Keywords: Adherence; Cystic fibrosis; Home spirometry; Telemedicine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
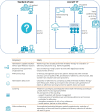
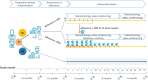
 = quarterly visit outpatient clinic,
= quarterly visit outpatient clinic,
 = intensive telephone coaching,
= intensive telephone coaching,
 telephone coaching,
telephone coaching,
 crisis phone call, t0 = inclusion into study, t1 = start assessment of adherence, t2 = start study intervention, t3 = six months of study intervention, t4 = 12 months of study intervention, t5 = end of study intervention
crisis phone call, t0 = inclusion into study, t1 = start assessment of adherence, t2 = start study intervention, t3 = six months of study intervention, t4 = 12 months of study intervention, t5 = end of study interventionReferences
-
- Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940–1948. doi: 10.1016/S0140-6736(19)32597-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

