Vascular Expression of Permeability-Resistant Occludin Mutant Preserves Visual Function in Diabetes
- PMID: 33883214
- PMCID: PMC8336002
- DOI: 10.2337/db20-1220
Vascular Expression of Permeability-Resistant Occludin Mutant Preserves Visual Function in Diabetes
Abstract
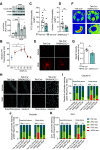
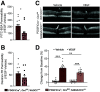
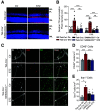
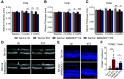
Diabetic retinopathy is one of the leading causes of vision loss and blindness. Extensive preclinical and clinical evidence exists for both vascular and neuronal pathology. However, the relationship of these changes in the neurovascular unit and impact on vision remains to be determined. Here, we investigate the role of tight junction protein occludin phosphorylation at S490 in modulating barrier properties and its impact on visual function. Conditional vascular expression of the phosphorylation-resistant Ser490 to Ala (S490A) form of occludin preserved tight junction organization and reduced vascular endothelial growth factor (VEGF)-induced permeability and edema formation after intraocular injection. In the retinas of streptozotocin-induced diabetic mice, endothelial-specific expression of the S490A form of occludin completely prevented diabetes-induced permeability to labeled dextran and inhibited leukostasis. Importantly, vascular-specific expression of the occludin mutant completely blocked the diabetes-induced decrease in visual acuity and contrast sensitivity. Together, these results reveal that occludin acts to regulate barrier properties downstream of VEGF in a phosphorylation-dependent manner and that loss of inner blood-retinal barrier integrity induced by diabetes contributes to vision loss.
© 2021 by the American Diabetes Association.
Figures
References
-
- Simó R, Sundstrom JM, Antonetti DA. Ocular Anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 2014;37:893–899 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

