Targeting resistance to radiation-immunotherapy in cold HNSCCs by modulating the Treg-dendritic cell axis
- PMID: 33883256
- PMCID: PMC8061827
- DOI: 10.1136/jitc-2020-001955
Targeting resistance to radiation-immunotherapy in cold HNSCCs by modulating the Treg-dendritic cell axis
Abstract
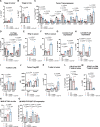
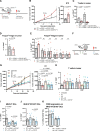
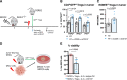
Background: Numerous trials combining radiation therapy (RT) and immunotherapy in head and neck squamous cell carcinoma (HNSCC) are failing. Using preclinical immune cold models of HNSCC resistant to RT-immune checkpoint inhibitors, we investigate therapeutic approaches of overcoming such resistance by examining the differential microenvironmental response to RT.
Methods: We subjected two HPV-negative orthotopic mouse models of HNSCC to combination RT, regulatory T cells (Treg) depletion, and/or CD137 agonism. Tumor growth was measured and intratumorous and lymph node immune populations were compared among treatment groups. Human gene sets, genetically engineered mouse models DEREG and BATF3-/-, flow and time-of-flight cytometry, RNA-Seq, Treg adoptive transfer studies, and in vitro experiments were used to further evaluate the role of dendritic cells (DCs) and Tregs in these treatments.
Results: In MOC2 orthotopic tumors, we find no therapeutic benefit to targeting classically defined immunosuppressive myeloids, which increase with RT. In these radioresistant tumors, supplementing combination RT and Treg depletion with anti-CD137 agonism stimulates CD103+ DC activation in tumor-draining lymph nodes as characterized by increases in CD80+ and CCR7+ DCs, resulting in a CD8 T cell-dependent response. Simultaneously, Tregs are reprogrammed to an effector phenotype demonstrated by increases in interferonγ+, tumor necrosis factorα+, PI3K+, pAKT+ and Eomes+ populations as well as decreases in CTLA4+ and NRP-1+ populations. Tumor eradication is observed when RT is increased to an 8 Gy x 5 hypofractionated regimen and combined with anti-CD25+ anti-CD137 treatment. In a human gene set from oral squamous cell carcinoma tumors, high Treg number is associated with earlier recurrence.
Conclusions: Regulating Treg functionality and DC activation status within the lymph node is critical for generating a T cell effector response in these highly radioresistant tumors. These findings underscore the plasticity of Tregs and represent a new therapeutic opportunity for reprogramming the tumor microenvironment in HNSCCs resistant to conventional radioimmunotherapy approaches.
Keywords: costimulatory and inhibitory t-cell receptors; dendritic cells; head and neck neoplasms; radioimmunotherapy; t-lymphocytes.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures




Similar articles
-
Complement C3a and C5a receptor blockade modulates regulatory T cell conversion in head and neck cancer.J Immunother Cancer. 2021 Mar;9(3):e002585. doi: 10.1136/jitc-2021-002585. J Immunother Cancer. 2021. PMID: 33789881 Free PMC article.
-
STAT3 Modulation of Regulatory T Cells in Response to Radiation Therapy in Head and Neck Cancer.J Natl Cancer Inst. 2019 Dec 1;111(12):1339-1349. doi: 10.1093/jnci/djz036. J Natl Cancer Inst. 2019. PMID: 30863843 Free PMC article.
-
Inhibition of the CCR6-CCL20 axis prevents regulatory T cell recruitment and sensitizes head and neck squamous cell carcinoma to radiation therapy.Cancer Immunol Immunother. 2023 May;72(5):1089-1102. doi: 10.1007/s00262-022-03313-2. Epub 2022 Nov 3. Cancer Immunol Immunother. 2023. PMID: 36326893 Free PMC article.
-
Dendritic cell-based immunotherapy for head and neck squamous cell carcinoma: advances and challenges.Front Immunol. 2025 May 26;16:1573635. doi: 10.3389/fimmu.2025.1573635. eCollection 2025. Front Immunol. 2025. PMID: 40491907 Free PMC article. Review.
-
The role of the tumor microenvironment in HNSCC resistance and targeted therapy.Front Immunol. 2025 Apr 1;16:1554835. doi: 10.3389/fimmu.2025.1554835. eCollection 2025. Front Immunol. 2025. PMID: 40236700 Free PMC article. Review.
Cited by
-
Mechanisms of radiotherapy resistance and radiosensitization strategies for esophageal squamous cell carcinoma.Mol Cancer. 2023 Aug 19;22(1):140. doi: 10.1186/s12943-023-01839-2. Mol Cancer. 2023. PMID: 37598158 Free PMC article. Review.
-
Mechanistic rationales for combining immunotherapy with radiotherapy.Front Immunol. 2023 Jun 12;14:1125905. doi: 10.3389/fimmu.2023.1125905. eCollection 2023. Front Immunol. 2023. PMID: 37377970 Free PMC article. Review.
-
Sensory nerve release of CGRP increases tumor growth in HNSCC by suppressing TILs.Med. 2024 Mar 8;5(3):254-270.e8. doi: 10.1016/j.medj.2024.02.002. Epub 2024 Feb 28. Med. 2024. PMID: 38423011 Free PMC article.
-
Dichotomous effects of cellular expression of STAT3 on tumor growth of HNSCC.Mol Ther. 2022 Mar 2;30(3):1149-1162. doi: 10.1016/j.ymthe.2021.11.011. Epub 2021 Nov 15. Mol Ther. 2022. PMID: 34793974 Free PMC article.
-
Remodeling the tumor microenvironment to overcome treatment resistance in HPV-negative head and neck cancer.Cancer Drug Resist. 2023 May 30;6(2):291-313. doi: 10.20517/cdr.2022.141. eCollection 2023. Cancer Drug Resist. 2023. PMID: 37457128 Free PMC article. Review.
References
-
- Diéras VC, Han HS, Kaufman B, et al. . Phase III study of veliparib with carboplatin and paclitaxel in HER2-negative advanced/metastatic gBRCA-associated breast cancer. Annals of Oncology 2019;30:v857–8. 10.1093/annonc/mdz394.008 - DOI
-
- Psyrri A, 910O – Cohen EE, Ferris RL . Primary results of the phase III JAVELIN head & neck 100 trial: Avelumab plus chemoradiotherapy (CRT) followed by avelumab maintenance vs CRT in patients with locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). ESMO Virtual Congress 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
