A Family of Toxoplasma gondii Genes Related to GRA12 Regulate Cyst Burdens and Cyst Reactivation
- PMID: 33883265
- PMCID: PMC8546695
- DOI: 10.1128/mSphere.00182-21
A Family of Toxoplasma gondii Genes Related to GRA12 Regulate Cyst Burdens and Cyst Reactivation
Abstract
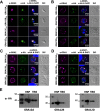
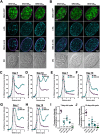
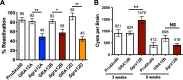
Toxoplasma gondii causes a chronic infection that renders the immunocompromised human host susceptible to toxoplasmic encephalitis triggered by cyst reactivation in the central nervous system. The dense granule protein GRA12 is a major parasite virulence factor required for parasite survival during acute infection. Here, we characterized the role of four GRA12-related genes in acute and chronic stages of infection. While GRA12A, GRA12B, and GRA12D were highly expressed in asexual stage tachyzoites and bradyzoites, expression of GRA12C appeared to be restricted to the sexual stages. In contrast to deletion of GRA12 (Δgra12), no major defects in acute virulence were observed in Δgra12A, Δgra12B, or Δgra12D parasites, though Δgra12B parasites exhibited an increased tachyzoite replication rate. Bradyzoites secreted GRA12A, GRA12B, and GRA12D and incorporated these molecules into the developing cyst wall, as well as the cyst matrix in distinct patterns. Similar to GRA12, GRA12A, GRA12B, and GRA12D colocalized with the dense granules in extracellular tachyzoites, with GRA2 and the intravacuolar network in the tachyzoite stage parasitophorous vacuole and with GRA2 in the cyst matrix and cyst wall. Chronic stage cyst burdens were decreased in mice infected with Δgra12A parasites and were increased in mice infected with Δgra12B parasites. However, Δgra12B cysts were not efficiently maintained in vivo Δgra12A, Δgra12B, and Δgra12D in vitro cysts displayed a reduced reactivation efficiency, and reactivation of Δgra12A cysts was delayed. Collectively, our results suggest that a family of genes related to GRA12 play significant roles in the formation, maintenance, and reactivation of chronic stage cysts.IMPORTANCE If host immunity weakens, Toxoplasma gondii cysts recrudesce in the central nervous system and cause a severe toxoplasmic encephalitis. Current therapies target acute stage infection but do not eliminate chronic cysts. Parasite molecules that mediate the development and persistence of chronic infection are poorly characterized. Dense granule (GRA) proteins such as GRA12 are key virulence factors during acute infection. Here, we investigated four GRA12-related genes. GRA12-related genes were not major virulence factors during acute infection. Instead, GRA12-related proteins localized at the cyst wall and cyst matrix and played significant roles in cyst development, persistence, and reactivation during chronic infection. Similar to GRA12, the GRA12-related proteins selectively associated with the intravacuolar network of membranes inside the vacuole. Collectively, our results support the hypothesis that GRA12 proteins associated with the intravacuolar membrane system support parasite virulence during acute infection and cyst development, persistence, and reactivation during chronic infection.
Keywords: GRA12; Toxoplasma gondii; bradyzoite differentiation; chronic infection; cyst development; cyst matrix; cyst persistence; cyst reactivation; cyst wall; cysts; dense granule; dense granules; intravacuolar network; virulence.
Copyright © 2021 Guevara et al.
Figures



Similar articles
-
Toxoplasma gondii Intravacuolar-Network-Associated Dense Granule Proteins Regulate Maturation of the Cyst Matrix and Cyst Wall.mSphere. 2019 Oct 16;4(5):e00487-19. doi: 10.1128/mSphere.00487-19. mSphere. 2019. PMID: 31619500 Free PMC article.
-
Toxoplasma gondii Parasitophorous Vacuole Membrane-Associated Dense Granule Proteins Orchestrate Chronic Infection and GRA12 Underpins Resistance to Host Gamma Interferon.mBio. 2019 Jul 2;10(4):e00589-19. doi: 10.1128/mBio.00589-19. mBio. 2019. PMID: 31266861 Free PMC article.
-
Toxoplasma gondii Parasitophorous Vacuole Membrane-Associated Dense Granule Proteins Regulate Maturation of the Cyst Wall.mSphere. 2020 Jan 15;5(1):e00851-19. doi: 10.1128/mSphere.00851-19. mSphere. 2020. PMID: 31941814 Free PMC article.
-
[Formation and diversity of parasitophorous vacuoles in parasitic protozoa. The Coccidia (Sporozoa, Apicomplexa)].Tsitologiia. 2003;45(4):339-56. Tsitologiia. 2003. PMID: 14520865 Review. Russian.
-
GRA proteins of Toxoplasma gondii: maintenance of host-parasite interactions across the parasitophorous vacuolar membrane.Korean J Parasitol. 2009 Oct;47 Suppl(Suppl):S29-37. doi: 10.3347/kjp.2009.47.S.S29. Korean J Parasitol. 2009. PMID: 19885333 Free PMC article. Review.
Cited by
-
GRA12 is a common virulence factor across Toxoplasma gondii strains and mouse subspecies.Nat Commun. 2025 Apr 16;16(1):3570. doi: 10.1038/s41467-025-58876-2. Nat Commun. 2025. PMID: 40240328 Free PMC article.
-
Human neutrophil-like cells demonstrate antimicrobial responses to the chronic cyst form of Toxoplasma gondii.Parasite Immunol. 2023 Dec;45(12):e13011. doi: 10.1111/pim.13011. Epub 2023 Sep 30. Parasite Immunol. 2023. PMID: 37776091 Free PMC article.
-
Functional Characterization of 15 Novel Dense Granule Proteins in Toxoplasma gondii Using the CRISPR-Cas9 System.Microbiol Spectr. 2023 Feb 14;11(1):e0307822. doi: 10.1128/spectrum.03078-22. Epub 2022 Dec 14. Microbiol Spectr. 2023. PMID: 36515555 Free PMC article.
-
Nanos gigantium humeris insidentes: old papers informing new research into Toxoplasma gondii.Int J Parasitol. 2021 Dec;51(13-14):1193-1212. doi: 10.1016/j.ijpara.2021.10.004. Epub 2021 Nov 1. Int J Parasitol. 2021. PMID: 34736901 Free PMC article. Review.
-
Toxoplasma gondii chitinase-like protein TgCLP1 regulates the parasite cyst burden.Front Cell Infect Microbiol. 2024 May 17;14:1359888. doi: 10.3389/fcimb.2024.1359888. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38828265 Free PMC article.
References
-
- McLeod R, Boyer KM, Lee D, Mui E, Wroblewski K, Karrison T, Noble AG, Withers S, Swisher CN, Heydemann PT, Sautter M, Babiarz J, Rabiah P, Meier P, Grigg ME, Toxoplasmosis Study Group . 2012. Prematurity and severity are associated with Toxoplasma gondii alleles (NCCCTS, 1981-2009). Clin Infect Dis 54:1595–1605. doi:10.1093/cid/cis258. - DOI - PMC - PubMed
-
- Hutson SL, Wheeler KM, McLone D, Frim D, Penn R, Swisher CN, Heydemann PT, Boyer KM, Noble AG, Rabiah P, Withers S, Montoya JG, Wroblewski K, Karrison T, Grigg ME, McLeod R. 2015. Patterns of hydrocephalus caused by congenital Toxoplasma gondii infection associate with parasite genetics. Clin Infect Dis 61:1831–1834. doi:10.1093/cid/civ720. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
