In vivo Multi-scale Photoacoustic Imaging Guided Photothermal Therapy of Cervical Cancer based on Customized Laser System and Targeted Nanoparticles
- PMID: 33883896
- PMCID: PMC8055284
- DOI: 10.2147/IJN.S301664
In vivo Multi-scale Photoacoustic Imaging Guided Photothermal Therapy of Cervical Cancer based on Customized Laser System and Targeted Nanoparticles
Abstract
Background: Effective treatment strategy for cervical carcinoma is subject to the limitation of its anatomical location and histological characteristics. Comprehensive imaging before cervical carcinoma treatment is of great significance for the patients. Current imaging methods cannot meet the requirements of high resolution, deep imaging depth and non-invasive imaging at the same time. Fortunately, Photoacoustic imaging (PAI) is a novel imaging method that combines rich optical contrast, high ultrasonic spatial resolution, and deep penetration depth in a single modality. Moreover, PAI-guided photothermal therapy (PTT) by aid of targeting nanoparticles is an emerging and effective cancer treatment in recent years.
Methods: Here, strong near-infrared region (NIR) absorption-conjugated polymer PIIGDTS (PD) nanoparticles with folic acid (FA) modification (namely, PD-FA) that targeted at Hela cell were specifically designed as cervical tumor imaging contrast agents and photothermal agents.
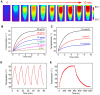
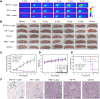
Results: The obtained PD-FA nanoparticles exhibited admirable photoacoustic contrast-enhancing ability and desirable PTT behavior with the photothermal conversion efficiency as high as 62.6% in vitro. Furthermore, the PAI performance and PTT efficiency were tested in HeLa tumor-bearing nude mice after injection of PD-FA nanoparticles. In vivo multi-scale, PAI provided B-san and 3D dimension imaging for intuitive and comprehensive information of Hela tumor. Moreover, the Hela tumor can be completely eliminated within 18 days after PTT, with no toxicity and side effects.
Conclusion: In summary, PD-FA injection combined with PAI and PTT systems provides a novel powerful tool for early diagnosis and precise treatment of cervical cancer.
Keywords: PIIGDTS nanoparticle; cervical cancer; multi-scale photoacoustic imaging; photothermal therapy.
© 2021 Qiu et al.
Conflict of interest statement
The authors reported no conflicts of interest for this work.
Figures







Similar articles
-
Noninvasive Photothermal Therapy of Nasopharyngeal Cancer Guided by High Efficiency Optical-Absorption Nanomaterial Enhanced by NIR-II Photoacoustic Imaging.Int J Nanomedicine. 2024 Jul 31;19:7817-7830. doi: 10.2147/IJN.S457069. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39099790 Free PMC article.
-
Targeted polydopamine nanoparticles enable photoacoustic imaging guided chemo-photothermal synergistic therapy of tumor.Acta Biomater. 2017 Jan 1;47:124-134. doi: 10.1016/j.actbio.2016.10.010. Epub 2016 Oct 6. Acta Biomater. 2017. PMID: 27721008
-
Folic acid-conjugated chitosan-functionalized graphene oxide for highly efficient photoacoustic imaging-guided tumor-targeted photothermal therapy.Int J Biol Macromol. 2020 Jul 15;155:961-971. doi: 10.1016/j.ijbiomac.2019.11.055. Epub 2019 Nov 9. Int J Biol Macromol. 2020. PMID: 31712157
-
Organic Semiconductors for Photothermal Therapy and Photoacoustic Imaging.Chembiochem. 2019 Jul 1;20(13):1628-1636. doi: 10.1002/cbic.201800818. Epub 2019 Apr 18. Chembiochem. 2019. PMID: 30690811 Review.
-
Photoacoustic Imaging and Photothermal Therapy of Semiconducting Polymer Nanoparticles: Signal Amplification and Second Near-Infrared Construction.Small. 2021 Feb;17(6):e2004723. doi: 10.1002/smll.202004723. Epub 2021 Jan 15. Small. 2021. PMID: 33448155 Review.
Cited by
-
Therapeutic and Contrast Agents for Photoacoustic Imaging-Guided Photothermal Therapy: A Narrative Review.Nanotheranostics. 2024 Aug 1;8(4):506-520. doi: 10.7150/ntno.96286. eCollection 2024. Nanotheranostics. 2024. PMID: 39135728 Free PMC article. Review.
-
Noninvasive Photothermal Therapy of Nasopharyngeal Cancer Guided by High Efficiency Optical-Absorption Nanomaterial Enhanced by NIR-II Photoacoustic Imaging.Int J Nanomedicine. 2024 Jul 31;19:7817-7830. doi: 10.2147/IJN.S457069. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39099790 Free PMC article.
-
Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review.Int J Nanomedicine. 2022 Dec 16;17:6427-6446. doi: 10.2147/IJN.S388996. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36540374 Free PMC article. Review.
-
Efficient Photoacoustic Imaging With Biomimetic Mesoporous Silica-Based Nanoparticles.Front Bioeng Biotechnol. 2021 Nov 30;9:762956. doi: 10.3389/fbioe.2021.762956. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34917596 Free PMC article.
-
Indocyanine Green-Based Theranostic Nanoplatform for NIR Fluorescence Image-Guided Chemo/Photothermal Therapy of Cervical Cancer.Int J Nanomedicine. 2021 Jul 17;16:4847-4861. doi: 10.2147/IJN.S318678. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34305398 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

