Calycosin Induces Gastric Cancer Cell Apoptosis via the ROS-Mediated MAPK/STAT3/NF-κB Pathway
- PMID: 33883905
- PMCID: PMC8053610
- DOI: 10.2147/OTT.S292388
Calycosin Induces Gastric Cancer Cell Apoptosis via the ROS-Mediated MAPK/STAT3/NF-κB Pathway
Abstract
Background: Calycosin, an active compound in plants, can promote the apoptosis of various cancer cells; however, the mechanism by which it regulates reactive oxygen species (ROS) in gastric cancer (GC) cells remains unclear.
Purpose: In this study, we investigated the effects of calycosin on apoptosis, the cell cycle, and migration in GC cells under ROS regulation.
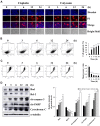
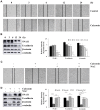
Results: The results of the Cell Counting Kit-8 assay suggested that calycosin had significant cytotoxic effects on 12 gastric cancer cells, but no significant cytotoxic effects on normal cells. Hoechst 33342/propidium iodide (PI) double staining and flow cytometry showed that calycosin had clear pro-apoptotic effects on AGS cells. Western blotting revealed that the expression of cytochrome C and pro-apoptotic proteins B-cell lymphoma 2 (Bcl-2)-associated agonist of cell death (Bad), cleaved (cle)-caspase-3, and cle-poly (ADP-ribose) polymerase gradually increased, and the expression of anti-apoptotic protein Bcl-2 gradually decreased. Calycosin also decreased the expression of extracellular signal-regulated kinase, nuclear factor kappa B (NF-κB), and signal transducer and activator of transcription 3 (STAT3), and increased the phosphorylation levels of p38, c-Jun N-terminal kinase, and inhibitor of NF-κB. In addition, calycosin markedly increased ROS accumulation, and pretreatment with active oxygen scavenger n-acetyl-l-cysteine (NAC) clearly inhibited apoptosis. Calycosin downregulated the cell cycle proteins cyclin-dependent kinase 2 (CDK2), CDK4, CDK6, cyclin D1, and cyclin E; upregulated p21 and p27; and arrested cells in the G0/G1 phase. Similarly, calycosin also downregulated Snail family transcriptional repressor 1, E-cadherin, and β-catenin and inhibited cell migration. However, pretreatment with NAC inhibited the calycosin-induced effects of cycle arrest and migration.
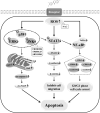
Conclusion: In summary, calycosin induces apoptosis via ROS-mediated MAPK/STAT3/NF-κB pathways, thereby exerting its anti-carcinogenic functions in GC cells.
Keywords: apoptosis; calycosin; cell cycle; cell migration; human gastric cancer; reactive oxygen species.
© 2021 Zhang et al.
Conflict of interest statement
The authors declare they have no conflicts of interest for this work.
Figures




Similar articles
-
Zeaxanthin Induces Apoptosis via ROS-Regulated MAPK and AKT Signaling Pathway in Human Gastric Cancer Cells.Onco Targets Ther. 2020 Oct 29;13:10995-11006. doi: 10.2147/OTT.S272514. eCollection 2020. Onco Targets Ther. 2020. PMID: 33149614 Free PMC article.
-
Calycosin induces mitochondrial-dependent apoptosis and cell cycle arrest, and inhibits cell migration through a ROS-mediated signaling pathway in HepG2 hepatocellular carcinoma cells.Toxicol In Vitro. 2021 Feb;70:105052. doi: 10.1016/j.tiv.2020.105052. Epub 2020 Nov 12. Toxicol In Vitro. 2021. PMID: 33188878
-
18β-Glycyrrhetinic Acid Has Anti-Cancer Effects via Inducing Apoptosis and G2/M Cell Cycle Arrest, and Inhibiting Migration of A549 Lung Cancer Cells.Onco Targets Ther. 2021 Oct 22;14:5131-5144. doi: 10.2147/OTT.S322852. eCollection 2021. Onco Targets Ther. 2021. PMID: 34712051 Free PMC article.
-
Neuropharmacological effects of calycosin: a translational review of molecular mechanisms and therapeutic applications.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr 16. doi: 10.1007/s00210-025-04154-3. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40237798 Review.
-
Mangiferin: An effective agent against human malignancies.Food Sci Nutr. 2024 Sep 8;12(10):7137-7157. doi: 10.1002/fsn3.4434. eCollection 2024 Oct. Food Sci Nutr. 2024. PMID: 39479608 Free PMC article. Review.
Cited by
-
Unveiling the potential anti-cancer activity of calycosin against multivarious cancers with molecular insights: A promising frontier in cancer research.Cancer Med. 2024 Feb;13(3):e6924. doi: 10.1002/cam4.6924. Epub 2024 Jan 17. Cancer Med. 2024. PMID: 38230908 Free PMC article. Review.
-
A review of the botany, phytochemistry, traditional uses, pharmacology, toxicology, and quality control of the Astragalus memeranaceus.Front Pharmacol. 2023 Aug 23;14:1242318. doi: 10.3389/fphar.2023.1242318. eCollection 2023. Front Pharmacol. 2023. PMID: 37680711 Free PMC article. Review.
-
Oxidative stress in the tumor microenvironment in gastric cancer and its potential role in immunotherapy.FEBS Open Bio. 2023 Jul;13(7):1238-1252. doi: 10.1002/2211-5463.13630. Epub 2023 May 20. FEBS Open Bio. 2023. PMID: 37171226 Free PMC article. Review.
-
Extraction, content determination, component analysis, and pharmacological action of Hedysari flavonoids: a review of research progress.Front Pharmacol. 2025 Apr 10;16:1517832. doi: 10.3389/fphar.2025.1517832. eCollection 2025. Front Pharmacol. 2025. PMID: 40276599 Free PMC article. Review.
-
TMJ-105, an extract of Carpesium cernuum, induced G2/M phase arrest and apoptosis via the JAK2/STAT3 axis and MAPKs signaling pathway in leukemia HEL cells.Heliyon. 2024 Jul 5;10(14):e34115. doi: 10.1016/j.heliyon.2024.e34115. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39108922 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

