Transcranial magnetic stimulation for the treatment of epilepsy
- PMID: 33884611
- PMCID: PMC8092469
- DOI: 10.1002/14651858.CD011025.pub3
Transcranial magnetic stimulation for the treatment of epilepsy
Abstract
Background: Epilepsy is a highly prevalent neurological condition characterised by repeated unprovoked seizures with various aetiologies. Although antiepileptic medications produce clinical improvement in many individuals, nearly a third of individuals have drug-resistant epilepsy that carries significant morbidity and mortality, and even individuals who have clinical improvement from antiepileptic medications often report iatrogenic symptoms. There remains a need for non-invasive and more effective therapies for this population. Transcranial magnetic stimulation (TMS) uses electromagnetic coils to excite or inhibit neurons, with repetitive pulses at low-frequency producing an inhibitory effect that could conceivably reduce cortical excitability associated with epilepsy. This is an updated version of the original Cochrane Review published in 2016.
Objectives: To assess the evidence for the use of TMS in individuals with drug-resistant epilepsy compared with other available treatments in reducing seizure frequency, improving quality of life, reducing epileptiform discharges, antiepileptic medication use, and side effects.
Search methods: For the latest update, we searched the Cochrane Register of Studies (CRS Web) and MEDLINE (Ovid 1946 to 2 June 2020). CRS Web includes randomised or quasi-randomised controlled trials from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the specialised registers of Cochrane Review Groups including Epilepsy.
Selection criteria: We included randomised controlled trials that were double-blinded, single-blinded, or unblinded, and placebo controlled, no treatment, or active controlled, which used repetitive transcranial magnetic stimulation (rTMS) without restriction of frequency, coil, duration or intensity on participants with drug-resistant epilepsy.
Data collection and analysis: We extracted information from each trial including methodological data; participant demographics including baseline seizure frequency, type of epileptic drugs taken; intervention details and intervention groups for comparison; potential biases; and outcomes and time points, primarily change in seizure frequency or responder rates, as well as quality of life and epileptiform discharges, adverse effects, and changes in medication use.
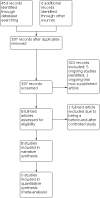
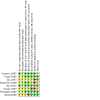
Main results: The original search revealed 274 records from the databases that after selection provided seven full-text relevant studies for inclusion. The latest search identified 179 new records from the databases that after evaluation against the inclusion and exclusion criteria provided one additional full-text relevant study. The eight included studies (241 participants) were all randomised trials; seven of the studies were blinded. Methodological and design information in the included studies was unclear, particularly relating to randomisation and allocation concealment methods. We were not able to combine the results of the trials in analysis due to differences in the studies' designs. For the current update, two of the eight studies analysed showed a statistically significant reduction in seizure rate from baseline (72% and 78.9% reduction of seizures per week from the baseline rate, respectively), whilst the other six studies showed no statistically significant difference in seizure frequency following rTMS treatment compared with controls (low-certainty evidence). One study assessed quality of life and found that more participants showed improvement in quality of life scores with active treatments compared to the sham treatment, but this only involved seven participants (very low-certainty evidence). Four studies evaluated our secondary endpoint of mean number of epileptic discharges, three of which showed a statistically significant reduction in discharges after active rTMS treatment. Adverse effects were uncommon in the studies and typically involved headache, dizziness, and tinnitus; however increased seizure frequency did occur in a small number of individuals. The included trials reported no significant changes in medication use. Overall the risk of bias was either low or unclear, and the certainty of the evidence was low to very low.
Authors' conclusions: Overall, we judged the certainty of evidence for the primary outcomes of this review to be low to very low. We found some evidence to suggest that rTMS is safe but some adverse events were experienced. The variability in technique and outcome reporting prevented meta-analysis, and the evidence for efficacy of rTMS for seizure reduction is still lacking, despite reasonable evidence that it is effective at reducing epileptiform discharges.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
DW: none known DS: none known SJN: none known BDM has received funding from UK Research and Innovation, Medical Research Council, National Institute for Health Research, Wellcome Trust, Academy of Medical Sciences, and British Medical Association.
Figures
Update of
-
Transcranial magnetic stimulation for the treatment of epilepsy.Cochrane Database Syst Rev. 2016 Aug 11;(8):CD011025. doi: 10.1002/14651858.CD011025.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Apr 15;4:CD011025. doi: 10.1002/14651858.CD011025.pub3. PMID: 27513825 Updated.
Similar articles
-
Transcranial magnetic stimulation for the treatment of epilepsy.Cochrane Database Syst Rev. 2016 Aug 11;(8):CD011025. doi: 10.1002/14651858.CD011025.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Apr 15;4:CD011025. doi: 10.1002/14651858.CD011025.pub3. PMID: 27513825 Updated.
-
Lamotrigine add-on therapy for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2020 Mar 20;3(3):CD001909. doi: 10.1002/14651858.CD001909.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Dec 11;12:CD001909. doi: 10.1002/14651858.CD001909.pub4. PMID: 32196639 Free PMC article. Updated.
-
Immediate antiepileptic drug treatment, versus placebo, deferred, or no treatment for first unprovoked seizure.Cochrane Database Syst Rev. 2021 May 4;5(5):CD007144. doi: 10.1002/14651858.CD007144.pub3. Cochrane Database Syst Rev. 2021. PMID: 33942281 Free PMC article.
-
Prophylactic drug management for febrile seizures in children.Cochrane Database Syst Rev. 2021 Jun 16;6(6):CD003031. doi: 10.1002/14651858.CD003031.pub4. Cochrane Database Syst Rev. 2021. PMID: 34131913 Free PMC article.
-
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2019 Jul 18;7(7):CD001911. doi: 10.1002/14651858.CD001911.pub4. Cochrane Database Syst Rev. 2019. PMID: 31318037 Free PMC article.
Cited by
-
Brain augmentation and neuroscience technologies: current applications, challenges, ethics and future prospects.Front Syst Neurosci. 2022 Sep 23;16:1000495. doi: 10.3389/fnsys.2022.1000495. eCollection 2022. Front Syst Neurosci. 2022. PMID: 36211589 Free PMC article. Review.
-
Investigating the Impact of Epilepsy on Cognitive Function: A Narrative Review.Cureus. 2023 Jun 30;15(6):e41223. doi: 10.7759/cureus.41223. eCollection 2023 Jun. Cureus. 2023. PMID: 37525802 Free PMC article. Review.
-
Navigating the depths: A comprehensive narrative review on depression in people with epilepsy.Heliyon. 2024 Dec 25;11(1):e41389. doi: 10.1016/j.heliyon.2024.e41389. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39845006 Free PMC article. Review.
-
Noninvasive brain stimulation as focal epilepsy treatment in the hospital, clinic, and home.Epilepsia Open. 2025 Jun;10(3):787-795. doi: 10.1002/epi4.70033. Epub 2025 Apr 10. Epilepsia Open. 2025. PMID: 40208019 Free PMC article.
-
Current evidence, clinical applications, and future directions of transcranial magnetic stimulation as a treatment for ischemic stroke.Front Neurosci. 2023 Jul 18;17:1177283. doi: 10.3389/fnins.2023.1177283. eCollection 2023. Front Neurosci. 2023. PMID: 37534033 Free PMC article. Review.
References
References to studies included in this review
Cantello 2007 {published data only}
-
- Cantello R, Rossi S, Varrasi C, Ulivelli M, Civardi C, Bartalini S, et al. Slow repetitive TMS for drug-resistant epilepsy: clinical and EEG findings of a placebo-controlled trial. Epilepsia 2007;48(2):366-74. - PubMed
Fregni 2006 {published data only}
-
- Fregni F, Otachi PT, Do Valle A, Boggio PS, Thut G, Rigonatti SP, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Annals of Neurology 2006;60(4):447-55. - PubMed
Joo 2007 {published data only}
-
- Joo E, Sun H, Chung S, Cho J, Seo D, Hong S. Antiepileptic effects of low-frequency repetitive transcranial magnetic stimulation by different stimulation durations and locations. Clinical Neurophysiology 2007;118(3):702-8. - PubMed
Seynaeve 2016 {published data only}
-
- Seynaeve L, Devroye A, Dupont P, Van Paesschen W. Randomized crossover sham-controlled clinical trial of targeted low-frequency transcranial magnetic stimulation comparing a figure-8 and a round coil to treat refractory neocortical epilepsy. Epilepsia 2016;57(1):141-50. [PMID: ] - PubMed
Sun 2012 {published data only}
-
- Sun W, Mao W, Meng X, Wang D, Qiao L, Tao W, et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: a controlled clinical study. Epilepsia 2012;53(10):1782-9. - PubMed
Tergau 2003 {published data only}
-
- Tergau F, Neumann D, Rosenow F, Nitsche MA, Paulus W, Steinhoff B. Can epilepsies be improved by repetitive transcranial magnetic stimulation? Interim analysis of a controlled study. Supplements to Clinical Neurophysiology 2003;56:400-5. - PubMed
Theodore 2002 {published data only}
-
- Theodore WH, Hunter K, Chen R, Vega-Bermudez F, Boroojerdi B, Reeves-Tyer P, et al. Transcranial magnetic stimulation for the treatment of seizures: a controlled study. Neurology 2002;59(4):560-2. - PubMed
Wang 2008 {published data only}
-
- Wang X, Yang D, Wang S, Zhao X, Zhang L, Chen Z, et al. Effects of low-frequency repetitive transcranial magnetic stimulation on electroencephalogram and seizure frequency in 15 patients with temporal lobe epilepsy following dipole source localization. Neural Regeneration Research 2008;3(11):1257-60.
References to studies excluded from this review
NCT00382707 {published data only}
-
- NCT00382707. Transcranial magnetic stimulation and anti-epileptic effect: optimization and evaluation with electrophysiology. clinicaltrials.gov/show/NCT00382707 (first received 29 September 2006).
References to ongoing studies
CTRI/2017/10/010067 {published data only (unpublished sought but not used)}
-
- CTRI/2017/10/010067. Experiment of magnetic stimulation of brain for treatment of fits in children. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=15419 (first received 12 October 2017).
CTRI/2019/02/017440 {published data only (unpublished sought but not used)}
-
- CTRI/2019/02/017440. Transcranial magnetic stimulation device for focal refractory epilepsy in childhood and adolescent. www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=30169 (first received 5 February 2019).
NCT02757547 {published data only (unpublished sought but not used)}
-
- NCT02757547. Transcranial magnetic stimulation for epilepsy. clinicaltrials.gov/show/nct02757547 (first received 2 May 2016).
NCT03154307 {published data only (unpublished sought but not used)}
-
- NCT03154307. Repeated TMS at low frequencies to reduce seizure occurrence. clinicaltrials.gov/show/nct03154307 (first received 16 May 2017).
Oberman 2015 {published data only (unpublished sought but not used)}
-
- Oberman L, Gersner R, Zangen A, Rotenberg A. Safety and tolerability of 1 HZ deep repetitive transcranial magnetic stimulation (RTMS) for treatment of temporal lobe epilepsy. Epilepsy Currents 2015;15(1):547.
Additional references
Atkins 2004
Cooper 2018
-
- Cooper YA, Pianka ST, Alotaibi NM, Babayan D, Salavati B, Weil AG, et al. Repetitive transcranial magnetic stimulation for the treatment of drug-resistant epilepsy: a systematic review and individual participant data meta-analysis of real-world evidence. Epilepsia Open 2018;3(1):55-65. - PMC - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Hsu 2011
-
- Hsu WY, Cheng CH, Lin MW, Shih YH, Liao KK, Lin YY. Antiepileptic effects of low frequency repetitive transcranial magnetic stimulation: a meta-analysis. Epilepsy Research 2011;96(3):231-40. - PubMed
Huang 2005
-
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 2005;45(2):201-6. - PubMed
Kimiskidis 2010
-
- Kimiskidis VK. Transcranial magnetic stimulation for drug-resistant epilepsies: rationale and clinical experience. European Neurology 2010;63(4):205-10. - PubMed
Kirkham 2010
-
- Kirkham J, Dwan K, Altman D, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. Research Methods and Reporting 2010;340:c365. - PubMed
Kwan 2011
-
- Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. New England Journal of Medicine 2011;365(10):919-26. - PubMed
Lefebvre 2020
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Moliadze 2003
NCT01745952
-
- NCT01745952. Multimodal image‐guided repetitive transcranial magnetic stimulation (rTMS) in the treatment of refractory partial epilepsy. clinicaltrials.gov/show/NCT01745952 (first received 10 December 2012). [CRSREF: 4352906]
Ngugi 2010
Ngugi 2011
Picot 2008
-
- Picot MC, Baldy-Moulinier M, Daurès JP, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia 2008;49(7):1230-8. - PubMed
Reithler 2011
-
- Reithler J, Peters JC, Sack AT. Multimodal transcranial magnetic stimulation: using concurrent neuroimaging to reveal the neural network dynamics of noninvasive brain stimulation. Progress in Neurobiology 2011;94(2):149-65. - PubMed
Ridding 2007
-
- Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nature Reviews Neuroscience 2007;8(7):559-67. - PubMed
Shorvon 2011
-
- Shorvon S. The causes of epilepsy: changing concepts of etiology of epilepsy over the past 150 years. Epilepsia 2011;52(6):1033-44. - PubMed
References to other published versions of this review
Chen 2014
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources