Cardiovascular Biomarkers and Heart Failure Risk in Stable Patients With Atherothrombotic Disease: A Nested Biomarker Study From TRA 2°P-TIMI 50
- PMID: 33884889
- PMCID: PMC8200769
- DOI: 10.1161/JAHA.120.018673
Cardiovascular Biomarkers and Heart Failure Risk in Stable Patients With Atherothrombotic Disease: A Nested Biomarker Study From TRA 2°P-TIMI 50
Abstract
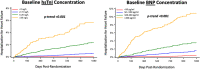
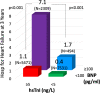
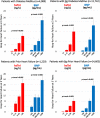
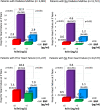
Background Patients with stable atherothrombotic disease vary in their risk of developing heart failure (HF). Circulating cardiovascular biomarkers may improve HF risk assessment and identify patients who may benefit from emerging HF preventive therapies. Methods and Results We measured high-sensitivity cardiac troponin I and BNP (B-type natriuretic peptide) in 15 833 patients with prior myocardial infarction, ischemic stroke, or peripheral artery disease from the TRA 2°P-TIMI 50 (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events-Thrombolysis in Myocardial Infarction 50) trial, excluding patients with recent myocardial infarction (<30 days). Biomarkers were categorized using a priori cut points. Hospitalization for HF (HHF) end points were adjudicated with blinded structured review of serious adverse events. Associations between biomarkers and HHF outcomes were adjusted for sex and independent clinical risk predictors of HHF in our cohort (age ≥75, prior HF, type 2 diabetes mellitus, polyvascular disease, body mass index, anemia, chronic kidney disease, hypertension). Baseline high-sensitivity cardiac troponin I and BNP each identified a significant graded risk of HHF independent of clinical risk predictors, including in the subgroups of patients with and without type 2 diabetes mellitus and with and without prior HF. Patients with both high-sensitivity cardiac troponin I ≥5 ng/L and BNP ≥100 pg/mL had the highest HHF event rates. When added to a multivariable Cox regression model with clinical risk predictors (C-index 0.88; 95% CI, 0.85-0.90), BNP (C -index 0.92; 95% CI, 0.90-0.93), and high-sensitivity cardiac troponin I (C-index 0.90; 95% CI, 0.88-0.92) each significantly improved the prognostic performance of the model (both PLRT<0.001). Conclusions Biomarkers of myocardial injury and hemodynamic stress are independent predictors of HHF risk in patients with stable atherothrombotic disease, with and without prior HF and/or type 2 diabetes mellitus. Registration URL: https://www.clinicaltrials.gov; Unique identifier: NCT00526474.
Keywords: atherosclerosis; biomarkers; heart failure.
Conflict of interest statement
Dr Berg, Dr Scirica, Ms. Goodrich, Dr Sabatine, and Dr Morrow are members of the TIMI Study Group, which has received institutional research grant support through Brigham and Women's Hospital from Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., Daiichi‐Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Inc., Roche, Siemens Healthcare Diagnostics, Inc., The Medicines Company, Zora Biosciences. Dr Freedman has no disclosures. Dr Bonaca reports receiving grant support from Amgen, AstraZeneca, Merck, Novo Nordisk, Pfizer, and Sanofi. Dr Jarolim has received research grants through his institution from Abbott Laboratories, Amgen, Inc, AstraZeneca, LP, Daiichi‐Sankyo, Inc, GlaxoSmithKline, Merck & Co, Inc, Roche Diagnostics Corporation, Takeda Global Research and Development Center, and Waters Technologies Corporation; and consulting fees from Roche Diagnostics Corporation. Dr Scirica has received consultant fees/honoraria from AbbVie, Allergan, Covance, Eisai, Elsevier Practice Update Cardiology, Esperion, Lexicon, Medtronic, NovoNordisk, Sanofi, AstraZeneca Pharmaceuticals, Biogen Idec, Boehringer Ingelheim Pharmaceuticals, Inc., Dr Reddy's Laboratories Inc., Forest Laboratories, GE Healthcare, GlaxoSmithKline, Health@Scale, Lexicon, Merck & Co., Inc., and St. Jude Medical; has received institutional research grants from AstraZeneca, Daiichi‐Sankyo, Eisai, Merck, Pfizer, and Poxel; and holds equity in Health@Scale. Dr Sabatine reports research grant support through Brigham and Women's Hospital from Amgen, Anthos Therapeutics, AstraZeneca, Bayer, Daiichi Sankyo, Eisai, Intarcia, The Medicines Company, MedImmune, Merck, Novartis, Pfizer, and Quark Pharmaceuticals and consulting fees from Althera, Amgen, Anthos Therapeutics, AstraZeneca, Bristol‐Myers Squibb, CVS Caremark, Dalcor, Dr. Reddy's Laboratories, Dynamix, IFM Therapeutics, Intarcia, The Medicines Company, MedImmune, and Merck. Dr Morrow has received grants and personal fees from Abbott Laboratories, AstraZeneca, Roche Diagnostics, and Bayer Pharma; grants from Novartis, Daiichi Sankyo, Eisai, GlaxoSmithKline, Takeda, Pfizer, Quark, The Medicines Company, Merck, and Zora Diagnostics; personal fees from InCarda.
Figures
References
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. DOI: 10.1093/eurheartj/ehw128. - DOI - PubMed
-
- Cheng S, Claggett B, Correia AW, Shah AM, Gupta DK, Skali H, Ni H, Rosamond WD, Heiss G, Folsom AR, et al. Temporal trends in the population attributable risk for cardiovascular disease: the Atherosclerosis Risk in Communities Study. Circulation. 2014;130:820–828. DOI: 10.1161/CIRCULATIONAHA.113.008506. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

