Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19: The TOGETHER Randomized Clinical Trial
- PMID: 33885775
- PMCID: PMC8063069
- DOI: 10.1001/jamanetworkopen.2021.6468
Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19: The TOGETHER Randomized Clinical Trial
Erratum in
-
Error in Visual Abstract.JAMA Netw Open. 2021 Sep 1;4(9):e2130442. doi: 10.1001/jamanetworkopen.2021.30442. JAMA Netw Open. 2021. PMID: 34533579 Free PMC article. No abstract available.
Abstract
Importance: Data on the efficacy of hydroxychloroquine or lopinavir-ritonavir for the treatment of high-risk outpatients with COVID-19 in developing countries are needed.
Objective: To determine whether hydroxychloroquine or lopinavir-ritonavir reduces hospitalization among high-risk patients with early symptomatic COVID-19 in an outpatient setting.
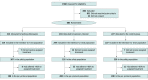
Design, setting, and participants: This randomized clinical trial was conducted in Brazil. Recently symptomatic adults diagnosed with respiratory symptoms from SARS-CoV-2 infection were enrolled between June 2 and September 30, 2020. The planned sample size was 1476 patients, with interim analyses planned after 500 patients were enrolled. The trial was stopped after the interim analysis for futility with a sample size of 685 patients. Statistical analysis was performed in December 2020.
Interventions: Patients were randomly assigned to hydroxychloroquine (800 mg loading dose, then 400 mg daily for 9 days), lopinavir-ritonavir (loading dose of 800 mg and 200 mg, respectively, every 12 hours followed by 400 mg and 100 mg, respectively, every 12 hours for the next 9 days), or placebo.
Main outcomes and measures: The primary outcomes were COVID-19-associated hospitalization and death assessed at 90 days after randomization. COVID-19-associated hospitalization was analyzed with a Cox proportional hazards model. The trial included the following secondary outcomes: all-cause hospitalization, viral clearance, symptom resolution, and adverse events.
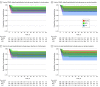
Results: Of 685 participants, 632 (92.3%) self-identified as mixed-race, 377 (55.0%) were women, and the median (range) age was 53 (18-94) years. A total of 214 participants were randomized to hydroxychloroquine; 244, lopinavir-ritonavir; and 227, placebo. At first interim analysis, the data safety monitoring board recommended stopping enrollment of both hydroxychloroquine and lopinavir-ritonavir groups because of futility. The proportion of patients hospitalized for COVID-19 was 3.7% (8 participants) in the hydroxychloroquine group, 5.7% (14 participants) in the lopinavir-ritonavir group, and 4.8% (11 participants) in the placebo group. We found no significant differences between interventions for COVID-19-associated hospitalization (hydroxychloroquine: hazard ratio [HR], 0.76 [95% CI, 0.30-1.88]; lopinavir-ritonavir: HR, 1.16 [95% CI, 0.53-2.56] as well as for the secondary outcome of viral clearance through day 14 (hydroxychloroquine: odds ratio [OR], 0.91 [95% CI, 0.82-1.02]; lopinavir-ritonavir: OR, 1.04 [95% CI, 0.94-1.16]). At the end of the trial, there were 3 fatalities recorded, 1 in the placebo group and 2 in the lopinavir-ritonavir intervention group.
Conclusions and relevance: In this randomized clinical trial, neither hydroxychloroquine nor lopinavir-ritonavir showed any significant benefit for decreasing COVID-19-associated hospitalization or other secondary clinical outcomes. This trial suggests that expedient clinical trials can be implemented in low-income settings even during the COVID-19 pandemic.
Trial registration: ClinicalTrials.gov Identifier: NCT04403100.
Conflict of interest statement
Figures
References
-
- Johns Hopkins University & Medicine . Coronavirus Resource Center. September 10, 2020. Accessed November 19, 2020. https://coronavirus.jhu.edu
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous