Efficacy and Safety of Certolizumab Pegol in Japanese Patients with Moderate to Severe Plaque Psoriasis: 52-Week Results
- PMID: 33886085
- PMCID: PMC8163922
- DOI: 10.1007/s13555-021-00520-0
Efficacy and Safety of Certolizumab Pegol in Japanese Patients with Moderate to Severe Plaque Psoriasis: 52-Week Results
Abstract
Introduction: Certolizumab pegol (CZP), an Fc-free, PEGylated anti-tumour necrosis factor biologic, dosed at 400 mg every 2 weeks (Q2W) and 200 mg Q2W over 16 weeks, resulted in improvements in Japanese patients with moderate to severe plaque psoriasis (PSO); no new safety signals were identified. We present 52-week efficacy and safety results.
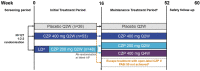
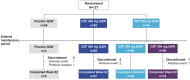
Methods: Patients ≥ 20 years with PSO ≥ 6 months [Psoriasis Area and Severity Index (PASI) ≥ 12, body surface area ≥ 10%, Physician's Global Assessment (PGA) ≥ 3] were randomised 1:2:2 to placebo Q2W, CZP 400 mg Q2W and CZP 200 mg Q2W (400 mg weeks 0/2/4) for 16 weeks. Week 16 PASI 50 responders continued through week 52; CZP 200 mg Q2W-randomised patients were re-randomised 1:1 to CZP 200 mg Q2W or CZP 400 mg Q4W; patients initially randomised to other treatment groups continued in the same group. Outcomes included PASI 75/90/100, PGA 0/1, Dermatology Life Quality Index (DLQI) 0/1, Itch Numeric Rating Scale (INRS) 0, modified Nail Psoriasis Severity Index (mNAPSI), durability of response for week 16 PASI 75/90 responders, and safety.
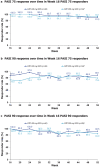
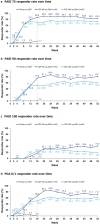
Results: Of 26/53/48 patients randomised to placebo, CZP 400 mg Q2W and CZP 200 mg Q2W, 2/47/39 completed week 52, respectively. PASI 75/90 responses were generally maintained from weeks 16 to 52 for all CZP doses. Most week 16 PASI 75/90 achievers maintained their response through week 52. PASI 75/90/100 responses at week 52 in the CZP 400 mg Q2W and CZP 200 mg Q2W groups were 83.0/81.1/41.5% and 72.9/60.4/18.8%, respectively; DLQI/INRS remission rates were 64.2/50.9% in CZP 400 mg Q2W and 58.3/27.1% in CZP 200 mg Q2W-treated patients. Reductions in mNAPSI observed for CZP-treated groups were maintained through week 52. No new safety signals were identified.
Conclusion: CZP treatment resulted in improvements in signs and symptoms of PSO, which were maintained through week 52. The 400 mg Q2W dose could provide additional clinical benefit.
Trial registration: NCT03051217.
Keywords: Anti-tumour necrosis factor; Certolizumab pegol; Japanese patients; Maintenance therapy; Plaque psoriasis.
Figures

References
-
- World Health Organization. Global report on Psoriasis. 2016. https://apps.who.int/iris/bitstream/handle/10665/204417/9789241565189_en.... Accessed 10 Dec 2020.
-
- Cassell SE, Bieber JD, Rich P, et al. The modified Nail Psoriasis Severity Index: validation of an instrument to assess psoriatic nail involvement in patients with psoriatic arthritis. J Rheumatol. 2007;34(1):123–129. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

