Strategies to Combat Multi-Drug Resistance in Tuberculosis
- PMID: 33886255
- PMCID: PMC8154215
- DOI: 10.1021/acs.accounts.0c00878
Strategies to Combat Multi-Drug Resistance in Tuberculosis
Abstract
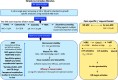
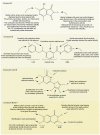
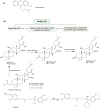
"Drug resistance is an unavoidable consequence of the use of drugs; however, the emergence of multi-drug resistance can be managed by accurate diagnosis and tailor-made regimens."Antimicrobial resistance (AMR), is one of the most paramount health perils that has emerged in the 21st century. The global increase in drug-resistant strains of various bacterial pathogens prompted the World Health Organization (WHO) to develop a priority list of AMR pathogens. Mycobacterium tuberculosis (Mtb), an acid-fast bacillus that causes tuberculosis (TB), merits being one of the highest priority pathogens on this list since drug-resistant TB (DR-TB) accounts for ∼29% of deaths attributable to AMR. In recent years, funded collaborative efforts of researchers from academia, not-for-profit virtual R&D organizations and industry have resulted in the continuous growth of the TB drug discovery and development pipeline. This has so far led to the accelerated regulatory approval of bedaquiline and delamanid for the treatment of DR-TB. However, despite the availability of drug regimes, the current cure rate for multi-drug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) treatment regimens is 50% and 30%, respectively. It is to be noted that these regimens are administered over a long duration and have a serious side effect profile. Coupled with poor patient adherence, this has led to further acquisition of drug resistance and treatment failure. There is therefore an urgent need to develop new TB drugs with novel mechanism of actions (MoAs) and associated regimens.This Account recapitulates drug resistance in TB, existing challenges in addressing DR-TB, new drugs and regimens in development, and potential ways to treat DR-TB. We highlight our research aimed at identifying novel small molecule leads and associated targets against TB toward contributing to the global TB drug discovery and development pipeline. Our work mainly involves screening of various small molecule chemical libraries in phenotypic whole-cell based assays to identify hits for medicinal chemistry optimization, with attendant deconvolution of the MoA. We discuss the identification of small molecule chemotypes active against Mtb and subsequent structure-activity relationships (SAR) and MoA deconvolution studies. This is followed by a discussion on a chemical series identified by whole-cell cross-screening against Mtb, for which MoA deconvolution studies revealed a pathway that explained the lack of in vivo efficacy in a mouse model of TB and reiterated the importance of selecting an appropriate growth medium during phenotypic screening. We also discuss our efforts on drug repositioning toward addressing DR-TB. In the concluding section, we preview some promising future directions and the challenges inherent in advancing the drug pipeline to address DR-TB.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- van der Westhuyzen R.; Winks S.; Wilson C. R.; Boyle G. A.; Gessner R. K.; Soares de Melo C.; Taylor D.; de Kock C.; Njoroge M.; Brunschwig C.; Lawrence N.; Rao S. P.; Sirgel F.; van Helden P.; Seldon R.; Moosa A.; Warner D. F.; Arista L.; Manjunatha U. H.; Smith P. W.; Street L. J.; Chibale K. Pyrrolo[3,4-c]pyridine-1,3(2H)-diones: A Novel Antimycobacterial Class Targeting Mycobacterial Respiration. J. Med. Chem. 2015, 58, 9371–9381. 10.1021/acs.jmedchem.5b01542. - DOI - PubMed
-
- Wilson C. R.; Gessner R. K.; Moosa A.; Seldon R.; Warner D. F.; Mizrahi V.; Soares de Melo C.; Simelane S. B.; Nchinda A.; Abay E.; Taylor D.; Njoroge M.; Brunschwig C.; Lawrence N.; Boshoff H. I. M.; Barry C. E. 3rd; Sirgel F. A.; van Helden P.; Harris C. J.; Gordon R.; Ghidelli-Disse S.; Pflaumer H.; Boesche M.; Drewes G.; Sanz O.; Santos G.; Rebollo-Lopez M. J.; Urones B.; Selenski C.; Lafuente-Monasterio M. J.; Axtman M.; Lelievre J.; Ballell L.; Mueller R.; Street L. J.; Ghorpade S. R.; Chibale K. Novel Antitubercular 6-Dialkylaminopyrimidine Carboxamides from Phenotypic Whole-Cell High Throughput Screening of a SoftFocus Library: Structure-Activity Relationship and Target Identification Studies. J. Med. Chem. 2017, 60, 10118–10134. 10.1021/acs.jmedchem.7b01347. - DOI - PMC - PubMed
-
- Soares de Melo C.; Singh V.; Myrick A.; Simelane S. B.; Taylor D.; Brunschwig C.; Lawrence N.; Schnappinger D.; Engelhart C. A.; Kumar A.; Parish T.; Su Q.; Myers T. G.; Boshoff H. I. M.; Barry C. E. 3rd; Sirgel F. A.; van Helden P. D.; Buchanan K. I.; Bayliss T.; Green S. R.; Ray P. C.; Wyatt P. G.; Basarab G. S.; Eyermann C. J.; Chibale K.; Ghorpade S. R. Antitubercular 2-Pyrazolylpyrimidinones: Structure-Activity Relationship and Mode-of-Action Studies. J. Med. Chem. 2021, 64, 719–740. 10.1021/acs.jmedchem.0c01727. - DOI - PMC - PubMed
-
- Akester J. N.; Njaria P.; Nchinda A.; Le Manach C.; Myrick A.; Singh V.; Lawrence N.; Njoroge M.; Taylor D.; Moosa A.; Smith A. J.; Brooks E. J.; Lenaerts A. J.; Robertson G. T.; Ioerger T. R.; Mueller R.; Chibale K. Synthesis, Structure–Activity Relationship, and Mechanistic Studies of Aminoquinazolinones Displaying Antimycobacterial Activity. ACS Infect. Dis. 2020, 6, 1951–1964. 10.1021/acsinfecdis.0c00252. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

