Binding Mode of Human Norepinephrine Transporter Interacting with HIV-1 Tat
- PMID: 33886267
- PMCID: PMC8562539
- DOI: 10.1021/acschemneuro.0c00792
Binding Mode of Human Norepinephrine Transporter Interacting with HIV-1 Tat
Abstract
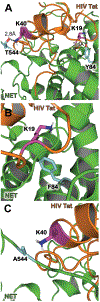
The increase of HIV infection in macrophages results in HIV proteins being released, like HIV Tat which impairs the function of monoamine transporters. HIV-infected patients have displayed increased synaptic levels of dopamine (DA) due to reduced binding and function of monoamine transporters such as the norepinephrine transporter (NET) and the dopamine transporter (DAT). Development of a three-dimensional model of the HIV-1 Tat-human NET (hNET) binding complex would help reveal how HIV-1 Tat causes toxicity in the neuron by affecting DA uptake. Here we use computational techniques such as molecular modeling to study microscopic properties and molecular dynamics of the HIV-1 Tat-hNET binding. These modeling techniques allow us to analyze noncovalent interactions and observe residue-residue contacts to verify a model structure. The modeling results studied here show that HIV-1 Tat-hNET binding is highly dynamic and that HIV-1 Tat preferentially binds to hNET in its outward-open state. In particular, HIV-1 Tat forms hydrogen bond interactions with side chains of hNET residues Y84, K88, and T544. The favorable hydrogen bonding interactions of HIV-1 Tat with the hNET side chain residues Y84 and T544 have been validated by our subsequently performed DA uptake activity assays and site-directed mutagenesis, suggesting that the modeled HIV-1 Tat-hNET binding mode is reasonable. These mechanistic and structural insights gained through homology models discussed in this study are expected to encourage the pursuit of pharmacological and biochemical studies on HIV-1 Tat interacting with hNET mechanisms and detailed structures.
Keywords: Molecular docking; dopamine; molecular dynamics; norepinephrine transporter; protein−protein interaction; trans-activator of transcription.
Figures

References
-
- Li W; Li G; Steiner J; Nath A, Role of Tat Protein in HIV Neuropathogenesis. Neurotoxicity Research 2009, 16 (3), 205–220. - PubMed
-
- Wallace DR; Dodson S; Nath A; Booze RM, Estrogen attenuates gp120- and tat1–72-induced oxidative stress and prevents loss of dopamine transporter function. Synapse 2006, 59 (1), 51–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

