Over half of clinical practice guidelines use non-systematic methods to inform recommendations: A methods study
- PMID: 33886670
- PMCID: PMC8062080
- DOI: 10.1371/journal.pone.0250356
Over half of clinical practice guidelines use non-systematic methods to inform recommendations: A methods study
Abstract
Introduction: Assessing the process used to synthesize the evidence in clinical practice guidelines enables users to determine the trustworthiness of the recommendations. Clinicians are increasingly dependent on guidelines to keep up with vast quantities of medical literature, and guidelines are followed to avoid malpractice suits. We aimed to assess whether systematic methods were used when synthesizing the evidence for guidelines; and to determine the type of review cited in support of recommendations.
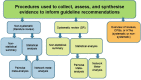
Methods: Guidelines published in 2017 and 2018 were retrieved from the TRIP and Epistemonikos databases. We randomly sorted and sequentially screened clinical guidelines on all topics to select the first 50 that met our inclusion criteria. Our primary outcomes were the number of guidelines using either a systematic or non-systematic process to gather, assess, and synthesise evidence; and the numbers of recommendations within guidelines based on different types of evidence synthesis (systematic or non-systematic reviews). If a review was cited, we looked for evidence that it was critically appraised, and recorded which quality assessment tool was used. Finally, we examined the relation between the use of the GRADE approach, systematic review process, and type of funder.
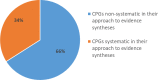
Results: Of the 50 guidelines, 17 (34%) systematically synthesised the evidence to inform recommendations. These 17 guidelines clearly reported their objectives and eligibility criteria, conducted comprehensive search strategies, and assessed the quality of the studies. Of the 29/50 guidelines that included reviews, 6 (21%) assessed the risk of bias of the review. The quality of primary studies was reported in 30/50 (60%) guidelines.
Conclusions: High quality, systematic review products provide the best available evidence to inform guideline recommendations. Using non-systematic methods compromises the validity and reliability of the evidence used to inform guideline recommendations, leading to potentially misleading and untrustworthy results.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Interventions for Age-Related Macular Degeneration: Are Practice Guidelines Based on Systematic Reviews?Ophthalmology. 2016 Apr;123(4):884-97. doi: 10.1016/j.ophtha.2015.12.004. Epub 2016 Jan 22. Ophthalmology. 2016. PMID: 26804762 Free PMC article.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Synthesis, grading, and presentation of evidence in guidelines: article 7 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report.Proc Am Thorac Soc. 2012 Dec;9(5):256-61. doi: 10.1513/pats.201208-060ST. Proc Am Thorac Soc. 2012. PMID: 23256168 Review.
-
Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome.Fertil Steril. 2018 Aug;110(3):364-379. doi: 10.1016/j.fertnstert.2018.05.004. Epub 2018 Jul 19. Fertil Steril. 2018. PMID: 30033227 Free PMC article. Review.
Cited by
-
Rigorous methodology and appropriate process are essential for high quality CPGs development.Int J Cardiol Heart Vasc. 2023 Mar 28;45:101197. doi: 10.1016/j.ijcha.2023.101197. eCollection 2023 Apr. Int J Cardiol Heart Vasc. 2023. PMID: 37070122 Free PMC article. No abstract available.
-
The optimal approach for retrieving systematic reviews was achieved when searching MEDLINE and Epistemonikos in addition to reference checking: a methodological validation study.BMC Med Res Methodol. 2024 Nov 9;24(1):271. doi: 10.1186/s12874-024-02384-2. BMC Med Res Methodol. 2024. PMID: 39522026 Free PMC article.
-
The Reporting and Methodological Quality of Systematic Reviews Underpinning Clinical Practice Guidelines Focused on the Management of Cutaneous Melanoma: Cross-Sectional Analysis.JMIR Dermatol. 2023 Dec 7;6:e43821. doi: 10.2196/43821. JMIR Dermatol. 2023. PMID: 38060306 Free PMC article.
-
A Systematic Review of Clinical Practice Guidelines for Neonatal Abstinence Syndrome.Children (Basel). 2023 Oct 13;10(10):1685. doi: 10.3390/children10101685. Children (Basel). 2023. PMID: 37892348 Free PMC article. Review.
-
Similarities, reliability and gaps in assessing the quality of conduct of systematic reviews using AMSTAR-2 and ROBIS: systematic survey of nutrition reviews.BMC Med Res Methodol. 2021 Nov 27;21(1):261. doi: 10.1186/s12874-021-01457-w. BMC Med Res Methodol. 2021. PMID: 34837960 Free PMC article.
References
-
- National Institute for Health and Clinical Excellence [Internet]. National Institute for Health and Clinical Excellence. Avilable from: http://www.nice.org.uk London, UK2019.
-
- NHMRC. Guidelines for Guidelines Handbook [Draft]. https://nhmrc.gov.au/guidelinesforguidelines. NSW, Australia: National Health and Medical Research Council, Australia Government; 2018.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

