Human Oxygenase Variants Employing a Single Protein FeII Ligand Are Catalytically Active
- PMID: 33887099
- PMCID: PMC8252765
- DOI: 10.1002/anie.202103711
Human Oxygenase Variants Employing a Single Protein FeII Ligand Are Catalytically Active
Abstract
Aspartate/asparagine-β-hydroxylase (AspH) is a human 2-oxoglutarate (2OG) and FeII oxygenase that catalyses C3 hydroxylations of aspartate/asparagine residues of epidermal growth factor-like domains (EGFDs). Unusually, AspH employs two histidine residues to chelate FeII rather than the typical triad of two histidine and one glutamate/aspartate residue. We report kinetic, inhibition, and crystallographic studies concerning human AspH variants in which either of its FeII binding histidine residues are substituted for alanine. Both the H725A and, in particular, the H679A AspH variants retain substantial catalytic activity. Crystal structures clearly reveal metal-ligation by only a single protein histidine ligand. The results have implications for the functional assignment of 2OG oxygenases and for the design of non-protein biomimetic catalysts.
Keywords: 2-oxoglutarate dependent oxygenase; aspartate/asparagine-β-hydroxylase; biomimetic catalysis; facial triad; metallo-enzymes.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


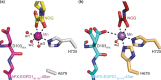
References
-
- None
-
- 2-Oxoglutarate-Dependent Oxygenases (Eds.: Schofield C. J., Hausinger R. P.), The Royal Society of Chemistry, Cambridge, 2015;
-
- None
-
- Kaelin W. G., Ratcliffe P. J., Mol. Cell 2008, 30, 393–402; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- C8717/A18245/CRUK_/Cancer Research UK/United Kingdom
- BB/M011224/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 106244/Z/14/Z/WT_/Wellcome Trust/United Kingdom
- 18245/CRUK_/Cancer Research UK/United Kingdom
- BB/J003018/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

