The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine
- PMID: 33887198
- PMCID: PMC8102360
- DOI: 10.1016/j.cmet.2021.03.025
The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine
Abstract
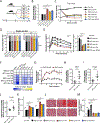
Low-protein diets promote metabolic health in rodents and humans, and the benefits of low-protein diets are recapitulated by specifically reducing dietary levels of the three branched-chain amino acids (BCAAs), leucine, isoleucine, and valine. Here, we demonstrate that each BCAA has distinct metabolic effects. A low isoleucine diet reprograms liver and adipose metabolism, increasing hepatic insulin sensitivity and ketogenesis and increasing energy expenditure, activating the FGF21-UCP1 axis. Reducing valine induces similar but more modest metabolic effects, whereas these effects are absent with low leucine. Reducing isoleucine or valine rapidly restores metabolic health to diet-induced obese mice. Finally, we demonstrate that variation in dietary isoleucine levels helps explain body mass index differences in humans. Our results reveal isoleucine as a key regulator of metabolic health and the adverse metabolic response to dietary BCAAs and suggest reducing dietary isoleucine as a new approach to treating and preventing obesity and diabetes.
Keywords: FGF21; GCN2; body mass index; branched-chain amino acids; diabetes; insulin resistance; isoleucine; mTORC1; obesity; valine.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests D.W.L. has received funding from and is a scientific advisory board member of Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. UW-Madison has applied for a patent based in part on the findings reported here, for which N.E.R. and D.W.L. are inventors.
Figures






Comment in
-
The coming of age for branched-chain amino acids.J Cardiovasc Aging. 2021;1(2):10.20517/jca.2021.02. doi: 10.20517/jca.2021.02. Epub 2021 May 14. J Cardiovasc Aging. 2021. PMID: 34568877 Free PMC article. No abstract available.
References
-
- (1999). Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation 100, 1132–1133. - PubMed
-
- Akter S, Mizoue T, Nanri A, Goto A, Noda M, Sawada N, Yamaji T, Iwasaki M, Inoue M, Tsugane S, et al. (2020). Low carbohydrate diet and all cause and cause-specific mortality. Clin Nutr. - PubMed
-
- Appuhamy JA, Knoebel NA, Nayananjalie WA, Escobar J, and Hanigan MD (2012). Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J Nutr 142, 484–491. - PubMed
-
- Arriola Apelo SI, Singer LM, Lin XY, McGilliard ML, St-Pierre NR, and Hanigan MD (2014). Isoleucine, leucine, methionine, and threonine effects on mammalian target of rapamycin signaling in mammary tissue. J Dairy Sci 97, 1047–1056. - PubMed
-
- Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, and Wasserman DH (2007). Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 56, 1025–1033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 AG066311/AG/NIA NIH HHS/United States
- I01 BX004031/BX/BLRD VA/United States
- R01 AG056771/AG/NIA NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- R21 AG061635/AG/NIA NIH HHS/United States
- R01 AG062328/AG/NIA NIH HHS/United States
- T32 AG000213/AG/NIA NIH HHS/United States
- RF1 AG056771/AG/NIA NIH HHS/United States
- P30 DK019525/DK/NIDDK NIH HHS/United States
- R56 AG056771/AG/NIA NIH HHS/United States
- P30 AR066524/AR/NIAMS NIH HHS/United States
- DP1 DK113643/DK/NIDDK NIH HHS/United States
- R01 GM113142/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

