Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization
- PMID: 33887205
- PMCID: PMC8053237
- DOI: 10.1016/j.chom.2021.04.007
Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization
Abstract
The emergence of SARS-CoV-2 variants has raised concerns about altered sensitivity to antibody-mediated immunity. The relative resistance of SARS-CoV-2 variants B.1.1.7 and B.1.351 to antibody neutralization has been recently investigated. We report that another emergent variant from Brazil, P.1, is not only refractory to multiple neutralizing monoclonal antibodies but also more resistant to neutralization by convalescent plasma and vaccinee sera. The magnitude of resistance is greater for monoclonal antibodies than vaccinee sera and evident with both pseudovirus and authentic P.1 virus. The cryoelectron microscopy structure of a soluble prefusion-stabilized spike reveals that the P.1 trimer adopts exclusively a conformation in which one of the receptor-binding domains is in the "up" position, which is known to facilitate binding to entry receptor ACE2. The functional impact of P.1 mutations thus appears to arise from local changes instead of global conformational alterations. The P.1 variant threatens current antibody therapies but less so protective vaccine efficacy.
Keywords: NTD; P.1; RBD; SARS-CoV-2; antibody; convalescent plasma; mutation; neutralization; vaccine; variant.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests P.W., J.Y., M.N., Y.H., L.L., and D.D.H. are inventors on a provisional patent application on mAbs to SARS-CoV-2.
Figures
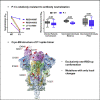

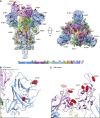
Update of
-
Increased Resistance of SARS-CoV-2 Variant P.1 to Antibody Neutralization.bioRxiv [Preprint]. 2021 Apr 9:2021.03.01.433466. doi: 10.1101/2021.03.01.433466. bioRxiv. 2021. Update in: Cell Host Microbe. 2021 May 12;29(5):747-751.e4. doi: 10.1016/j.chom.2021.04.007. PMID: 33688656 Free PMC article. Updated. Preprint.
References
-
- Adams P.D., Gopal K., Grosse-Kunstleve R.W., Hung L.W., Ioerger T.R., McCoy A.J., Moriarty N.W., Pai R.K., Read R.J., Romo T.D., et al. Recent developments in the PHENIX software for automated crystallographic structure determination. J. Synchrotron Radiat. 2004;11:53–55. - PubMed
-
- Annavajhala M.K., Mohri H., Zucker J.E., Sheng Z., Wang P., Gomez-Simmonds A., Ho D.D., Uhlemann A.-C. A Novel SARS-CoV-2 Variant of Concern, B.1.526, Identified in New York. medRxiv. 2021 doi: 10.1101/2021.02.23.21252259. - DOI
-
- Cerutti G., Guo Y., Zhou T., Gorman J., Lee M., Rapp M., Reddem E.R., Yu J., Bahna F., Bimela J., et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe. 2021 j.chom.2021.03.005 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

