Synthesis, Molecular Pharmacology, and Structure-Activity Relationships of 3-(Indanoyl)indoles as Selective Cannabinoid Type 2 Receptor Antagonists
- PMID: 33887913
- PMCID: PMC8683641
- DOI: 10.1021/acs.jmedchem.1c00442
Synthesis, Molecular Pharmacology, and Structure-Activity Relationships of 3-(Indanoyl)indoles as Selective Cannabinoid Type 2 Receptor Antagonists
Abstract
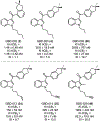
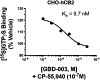
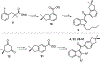
Synthetic indole cannabinoids characterized by a 2',2'-dimethylindan-5'-oyl group at the indole C3 position constitute a new class of ligands possessing high affinity for human CB2 receptors at a nanomolar concentration and a good selectivity index. Starting from the neutral antagonist 4, the effects of indole core modification on the pharmacodynamic profile of the ligands were investigated. Several N1 side chains afforded potent and CB2-selective neutral antagonists, notably derivatives 26 (R1 = n-propyl, R2 = H) and 35 (R1 = 4-pentynyl, R2 = H). Addition of a methyl group at C2 improved the selectivity for the CB2 receptor. Moreover, C2 indole substitution may control the CB2 activity as shown by the functionality switch in 35 (antagonist) and 49 (R1 = 4-pentynyl, R2 = CH3, partial agonist).
Figures


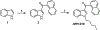
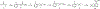


Similar articles
-
7-Azaindolequinuclidinones (7-AIQD): A novel class of cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptor ligands.Bioorg Med Chem Lett. 2020 Nov 15;30(22):127501. doi: 10.1016/j.bmcl.2020.127501. Epub 2020 Aug 31. Bioorg Med Chem Lett. 2020. PMID: 32882418 Free PMC article.
-
Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity.J Med Chem. 2010 Jan 14;53(1):295-315. doi: 10.1021/jm901214q. J Med Chem. 2010. PMID: 19921781
-
Strategies to develop selective CB2 receptor agonists from indole carboxamide synthetic cannabinoids.Eur J Med Chem. 2019 Oct 15;180:291-309. doi: 10.1016/j.ejmech.2019.07.036. Epub 2019 Jul 11. Eur J Med Chem. 2019. PMID: 31319265
-
CB2 receptor ligands.Mini Rev Med Chem. 2005 Jul;5(7):641-9. doi: 10.2174/1389557054368844. Mini Rev Med Chem. 2005. PMID: 16026310 Review.
-
Targeting the cannabinoid CB2 receptor: modelling and structural determinants of CB2 selective ligands.Br J Pharmacol. 2008 Jan;153(2):335-46. doi: 10.1038/sj.bjp.0707567. Epub 2007 Nov 5. Br J Pharmacol. 2008. PMID: 17982473 Free PMC article. Review.
Cited by
-
Rational drug design of CB2 receptor ligands: from 2012 to 2021.RSC Adv. 2022 Dec 8;12(54):35242-35259. doi: 10.1039/d2ra05661e. eCollection 2022 Dec 6. RSC Adv. 2022. PMID: 36540233 Free PMC article. Review.
-
Photoinduced ynamide structural reshuffling and functionalization.Nat Commun. 2022 Apr 29;13(1):2345. doi: 10.1038/s41467-022-30001-7. Nat Commun. 2022. PMID: 35487916 Free PMC article.
-
Synthesis, Structure-Activity Relationships, Radiofluorination, and Biological Evaluation of [18F]RM365, a Novel Radioligand for Imaging the Human Cannabinoid Receptor Type 2 (CB2R) in the Brain with PET.J Med Chem. 2023 Oct 26;66(20):13991-14010. doi: 10.1021/acs.jmedchem.3c01035. Epub 2023 Oct 10. J Med Chem. 2023. PMID: 37816245 Free PMC article.
-
Benzannulation and Hydrocarboxylation Methods for the Synthesis of a Neopentylene-Fused Analogue of Ibuprofen.ACS Omega. 2021 Oct 26;6(44):30108-30114. doi: 10.1021/acsomega.1c04943. eCollection 2021 Nov 9. ACS Omega. 2021. PMID: 34778682 Free PMC article.
References
-
- Di Marzo V Targeting the Endocannabinoid System: To Enhance or Reduce? Nat. Rev. Drug Discovery 2008, 7, 438–455. - PubMed
-
- Finn DP Endocannabinoid-mediated Modulation of Stress Responses: Physiological and Pathophysiological Significance. Immunobiology 2010, 215, 629–646. - PubMed
-
- Oesch S; Gertsch J Cannabinoid Receptor Ligands as Potential Anticancer Agents—High Hopes for New Therapies? J. Pharm. Pharmacol 2009, 61, 839–853. - PubMed
-
- Karsak M; Gaffal E; Date R; Wang-Eckhardt L; Rehnelt J; Petrosino S; Starowicz K; Steuder R; Schlicker E; Cravatt B; Mechoulam R; Buettner R; Werner S; Di Marzo V; Tuting T; Zimmer A Attenuation of Allergic Contact Dermatitis Through the Endocannabinoid System. Science 2007, 316, 1494–1497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

