Unraveling Deep Gray Matter Atrophy and Iron and Myelin Changes in Multiple Sclerosis
- PMID: 33888456
- PMCID: PMC8324266
- DOI: 10.3174/ajnr.A7093
Unraveling Deep Gray Matter Atrophy and Iron and Myelin Changes in Multiple Sclerosis
Abstract
Background and purpose: Modifications of magnetic susceptibility have been consistently demonstrated in the subcortical gray matter of MS patients, but some uncertainties remain concerning the underlying neurobiological processes and their clinical relevance. We applied quantitative susceptibility mapping and longitudinal relaxation rate relaxometry to clarify the relative contribution of atrophy and iron and myelin changes to deep gray matter damage and disability in MS.
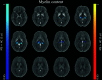
Materials and methods: Quantitative susceptibility mapping and longitudinal relaxation rate maps were computed for 91 patients and 55 healthy controls from MR images acquired at 3T. Applying an external model, we estimated iron and myelin concentration maps for all subjects. Subsequently, changes of deep gray matter iron and myelin concentration (atrophy-dependent) and content (atrophy-independent) were investigated globally (bulk analysis) and regionally (voxel-based and atlas-based thalamic subnuclei analyses). The clinical impact of the observed MRI modifications was evaluated via regression models.
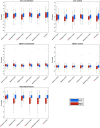
Results: We identified reduced thalamic (P < .001) and increased pallidal (P < .001) mean iron concentrations in patients with MS versus controls. Global myelin and iron content in the basal ganglia did not differ between the two groups, while actual iron depletion was present in the thalamus (P < .001). Regionally, patients showed increased iron concentration in the basal ganglia (P ≤ .001) and reduced iron and myelin content in thalamic posterior-medial regions (P ≤ .004), particularly in the pulvinar (P ≤ .001). Disability was predicted by thalamic volume (B = -0.341, P = .02), iron concentration (B = -0.379, P = .005) and content (B = -0.406, P = .009), as well as pulvinar iron (B = -0.415, P = .003) and myelin (B = -0.415, P = .02) content, independent of atrophy.
Conclusions: Quantitative MRI suggests an atrophy-related iron increase within the basal ganglia of patients with MS, along with an atrophy-independent reduction of thalamic iron and myelin correlating with disability. Absolute depletions of thalamic iron and myelin may represent sensitive markers of subcortical GM damage, which add to the clinical impact of thalamic atrophy in MS.
© 2021 by American Journal of Neuroradiology.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
