The roles and regulation of MDM2 and MDMX: it is not just about p53
- PMID: 33888565
- PMCID: PMC8091979
- DOI: 10.1101/gad.347872.120
The roles and regulation of MDM2 and MDMX: it is not just about p53
Abstract
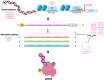
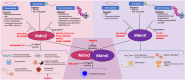
Most well studied as proteins that restrain the p53 tumor suppressor protein, MDM2 and MDMX have rich lives outside of their relationship to p53. There is much to learn about how these two proteins are regulated and how they can function in cells that lack p53. Regulation of MDM2 and MDMX, which takes place at the level of transcription, post-transcription, and protein modification, can be very intricate and is context-dependent. Equally complex are the myriad roles that these two proteins play in cells that lack wild-type p53; while many of these independent outcomes are consistent with oncogenic transformation, in some settings their functions could also be tumor suppressive. Since numerous small molecules that affect MDM2 and MDMX have been developed for therapeutic outcomes, most if not all designed to prevent their restraint of p53, it will be essential to understand how these diverse molecules might affect the p53-independent activities of MDM2 and MDMX.
Keywords: MDM2; MDM2 and MDMX inhibitors; MDMX; cancer; p53; pathologies; roles; upstream regulation.
© 2021 Klein et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
