Thermosensitive TRPV4 channels mediate temperature-dependent microglia movement
- PMID: 33888579
- PMCID: PMC8092382
- DOI: 10.1073/pnas.2012894118
Thermosensitive TRPV4 channels mediate temperature-dependent microglia movement
Abstract
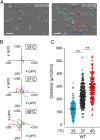
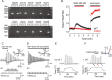
Microglia maintain central nervous system homeostasis by monitoring changes in their environment (resting state) and by taking protective actions to equilibrate such changes (activated state). These surveillance and protective roles both require constant movement of microglia. Interestingly, induced hypothermia can reduce microglia migration caused by ischemia, suggesting that microglia movement can be modulated by temperature. Although several ion channels and transporters are known to support microglia movement, the precise molecular mechanism that regulates temperature-dependent movement of microglia remains unclear. Some members of the transient receptor potential (TRP) channel superfamily exhibit thermosensitivity and thus are strong candidates for mediation of this phenomenon. Here, we demonstrate that mouse microglia exhibit temperature-dependent movement in vitro and in vivo that is mediated by TRPV4 channels within the physiological range of body temperature. Our findings may provide a basis for future research into the potential clinical application of temperature regulation to preserve cell function via manipulation of ion channel activity.
Keywords: TRP channels; TRPV4; microglia; movement.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Nimmerjahn A., Kirchhoff F., Helmchen F., Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005). - PubMed
-
- Wolf S. A., Boddeke H. W., Kettenmann H., Microglia in physiology and disease. Annu. Rev. Physiol. 79, 619–643 (2017). - PubMed
-
- Yenari M. A., Han H. S., Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 13, 267–278 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

