Cholinergic neurons constitutively engage the ISR for dopamine modulation and skill learning in mice
- PMID: 33888613
- PMCID: PMC8457366
- DOI: 10.1126/science.abe1931
Cholinergic neurons constitutively engage the ISR for dopamine modulation and skill learning in mice
Abstract
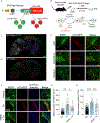
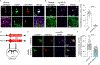
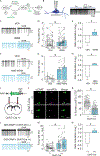
The integrated stress response (ISR) maintains proteostasis by modulating protein synthesis and is important in synaptic plasticity, learning, and memory. We developed a reporter, SPOTlight, for brainwide imaging of ISR state with cellular resolution. Unexpectedly, we found a class of neurons in mouse brain, striatal cholinergic interneurons (CINs), in which the ISR was activated at steady state. Genetic and pharmacological manipulations revealed that ISR signaling was necessary in CINs for normal type 2 dopamine receptor (D2R) modulation. Inhibiting the ISR inverted the sign of D2R modulation of CIN firing and evoked dopamine release and altered skill learning. Thus, a noncanonical, steady-state mode of ISR activation is found in CINs, revealing a neuromodulatory role for the ISR in learning.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


Comment in
-
A spotlight on the elusive striatal cholinergic interneuron.Science. 2021 Apr 23;372(6540):345-346. doi: 10.1126/science.abi4907. Science. 2021. PMID: 33888628 Free PMC article. No abstract available.
-
Stressing the Importance of Cholinergic Interneurons in Striatal Function.Mov Disord. 2022 Jan;37(1):36. doi: 10.1002/mds.28869. Epub 2021 Nov 22. Mov Disord. 2022. PMID: 34806801 Free PMC article. No abstract available.
References
-
- Harding HP et al. , An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

