Transcriptomic analysis identifies differences in gene expression in actinic keratoses after treatment with imiquimod and between responders and non responders
- PMID: 33888854
- PMCID: PMC8062619
- DOI: 10.1038/s41598-021-88424-z
Transcriptomic analysis identifies differences in gene expression in actinic keratoses after treatment with imiquimod and between responders and non responders
Abstract
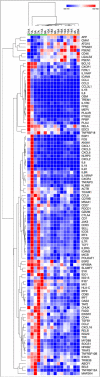
The presence of actinic keratoses (AKs) increases a patient's risk of developing squamous cell carcinoma by greater than six-fold. We evaluated the effect of topical treatment with imiquimod on the tumor microenvironment by measuring transcriptomic differences in AKs before and after treatment with imiquimod 3.75%. Biopsies were collected prospectively from 21 patients and examined histologically. RNA was extracted and transcriptomic analyses of 788 genes were performed using the nanoString assay. Imiquimod decreased number of AKs by study endpoint at week 14 (p < 0.0001). Post-imiquimod therapy, levels of CDK1, CXCL13, IL1B, GADPH, TTK, ILF3, EWSR1, BIRC5, PLAUR, ISG20, and C1QBP were significantly lower (adjusted p < 0.05). Complete responders (CR) exhibited a distinct pattern of inflammatory gene expression pre-treatment relative to incomplete responders (IR), with alterations in 15 inflammatory pathways (p < 0.05) reflecting differential expression of 103 genes (p < 0.05). Presence of adverse effects was associated with improved treatment response. Differences in gene expression were found between pre-treatment samples in CR versus IR, suggesting that higher levels of inflammation pre-treament may play a part in regression of AKs. Further characterization of the immune micro-environment in AKs may help develop biomarkers predictive of response to topical immune modulators and may guide therapy.
Conflict of interest statement
Yvonne Saenger receives funding from Amgen and Regeneron and has equity in a small biomarker start-up company, Wasaba. The remaining authors have no relevant conflicts of interest to disclose.
Figures



References
-
- The Lewin Group, I. The Burden of Skin Disease 2005. Prepared for the Society for investigative Dermatology.
-
- Lee, P. K. in Clinical Dermatology (eds Carol Soutor & Maria K. Hordinsky) (McGraw-Hill Education, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

