Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells
- PMID: 33888899
- PMCID: PMC8451032
- DOI: 10.1038/s41591-021-01326-5
Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells
Abstract
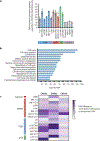
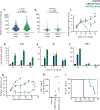
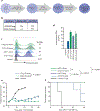
While CD19-directed chimeric antigen receptor (CAR) T cells can induce remission in patients with B cell acute lymphoblastic leukemia (ALL), a large subset relapse with CD19- disease. Like CD19, CD22 is broadly expressed by B-lineage cells and thus serves as an alternative immunotherapy target in ALL. Here we present the composite outcomes of two pilot clinical trials ( NCT02588456 and NCT02650414 ) of T cells bearing a 4-1BB-based, CD22-targeting CAR in patients with relapsed or refractory ALL. The primary end point of these studies was to assess safety, and the secondary end point was antileukemic efficacy. We observed unexpectedly low response rates, prompting us to perform detailed interrogation of the responsible CAR biology. We found that shortening of the amino acid linker connecting the variable heavy and light chains of the CAR antigen-binding domain drove receptor homodimerization and antigen-independent signaling. In contrast to CD28-based CARs, autonomously signaling 4-1BB-based CARs demonstrated enhanced immune synapse formation, activation of pro-inflammatory genes and superior effector function. We validated this association between autonomous signaling and enhanced function in several CAR constructs and, on the basis of these observations, designed a new short-linker CD22 single-chain variable fragment for clinical evaluation. Our findings both suggest that tonic 4-1BB-based signaling is beneficial to CAR function and demonstrate the utility of bedside-to-bench-to-bedside translation in the design and implementation of CAR T cell therapies.
Figures


Comment in
-
Context matters in CAR T cell tonic signaling.Nat Med. 2021 May;27(5):763-764. doi: 10.1038/s41591-021-01340-7. Nat Med. 2021. PMID: 33972792 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R01 CA226983/CA/NCI NIH HHS/United States
- P01 CA214278/CA/NCI NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- R21 HL125018/HL/NHLBI NIH HHS/United States
- U01 CA232361/CA/NCI NIH HHS/United States
- R56 AI130197/AI/NIAID NIH HHS/United States
- K99 CA212302/CA/NCI NIH HHS/United States
- K08 CA194256/CA/NCI NIH HHS/United States
- R01 GM104867/GM/NIGMS NIH HHS/United States
- R01 AI130197/AI/NIAID NIH HHS/United States
- R00 CA212302/CA/NCI NIH HHS/United States
- R21 AI129594/AI/NIAID NIH HHS/United States
- T32 CA009140/CA/NCI NIH HHS/United States
- R21 AI124769/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

