Experimental Drugs for the Treatment of Hepatitis D
- PMID: 33889032
- PMCID: PMC8057838
- DOI: 10.2147/JEP.S235550
Experimental Drugs for the Treatment of Hepatitis D
Abstract
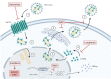
Chronic hepatitis D virus infection is the most severe form of viral hepatitis. Antiviral treatment is urgently needed to prevent patients from developing end stage liver disease or hepatocellular carcinoma. Treatment options were limited to off-label use of pegylated interferon alfa until conditional approval of bulevirtide by the EMA (European Medicines Agency) in July 2020. However, several other antiviral compounds are currently investigated and represent promising agents for the treatment of chronic HDV infection.
Keywords: HBV/HDV coinfection; bulevirtide; hepatitis delta; interferon; lonafarnib.
© 2021 Sandmann and Cornberg.
Conflict of interest statement
MC has received personal fees from AbbVie, Gilead Sciences, GSK, Janssen-Cilag, MSD Sharp & Dohme, Novartis, Spring Bank Pharmaceuticals, Swedish Orphan Biovitrum AB (SOBI). The authors report no other conflicts of interest in this work.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

