The Emerging Role of the Interplay Among Astrocytes, Microglia, and Neurons in the Hippocampus in Health and Disease
- PMID: 33889084
- PMCID: PMC8055856
- DOI: 10.3389/fnagi.2021.651973
The Emerging Role of the Interplay Among Astrocytes, Microglia, and Neurons in the Hippocampus in Health and Disease
Abstract
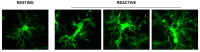
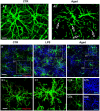
For over a century, neurons have been considered the basic functional units of the brain while glia only elements of support. Activation of glia has been long regarded detrimental for survival of neurons but more it appears that this is not the case in all circumstances. In this review, we report and discuss the recent literature on the alterations of astrocytes and microglia during inflammaging, the low-grade, slow, chronic inflammatory response that characterizes normal brain aging, and in acute inflammation. Becoming reactive, astrocytes and microglia undergo transcriptional, functional, and morphological changes that transform them into cells with different properties and functions, such as A1 and A2 astrocytes, and M1 and M2 microglia. This classification of microglia and astrocytes in two different, all-or-none states seems too simplistic, and does not correspond to the diverse variety of phenotypes so far found in the brain. Different interactions occur among the many cell populations of the central nervous system in health and disease conditions. Such interactions give rise to networks of morphological and functional reciprocal reliance and dependency. Alterations affecting one cell population reverberate to the others, favoring or dysregulating their activities. In the last part of this review, we present the modifications of the interplay between neurons and glia in rat models of brain aging and acute inflammation, focusing on the differences between CA1 and CA3 areas of the hippocampus, one of the brain regions most susceptible to different insults. With triple labeling fluorescent immunohistochemistry and confocal microscopy (TIC), it is possible to evaluate and compare quantitatively the morphological and functional alterations of the components of the neuron-astrocyte-microglia triad. In the contiguous and interconnected regions of rat hippocampus, CA1 and CA3 Stratum Radiatum, astrocytes and microglia show a different, finely regulated, and region-specific reactivity, demonstrating that glia responses vary in a significant manner from area to area. It will be of great interest to verify whether these differential reactivities of glia explain the diverse vulnerability of the hippocampal areas to aging or to different damaging insults, and particularly the higher sensitivity of CA1 pyramidal neurons to inflammatory stimuli.
Keywords: CA1; CA3; acute inflammation; confocal microscopy; inflammaging; neurodegeneration; triads.
Copyright © 2021 Lana, Ugolini, Nosi, Wenk and Giovannini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
An Overview on the Differential Interplay Among Neurons-Astrocytes-Microglia in CA1 and CA3 Hippocampus in Hypoxia/Ischemia.Front Cell Neurosci. 2020 Nov 11;14:585833. doi: 10.3389/fncel.2020.585833. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33262692 Free PMC article. Review.
-
Phenomic Microglia Diversity as a Druggable Target in the Hippocampus in Neurodegenerative Diseases.Int J Mol Sci. 2023 Sep 5;24(18):13668. doi: 10.3390/ijms241813668. Int J Mol Sci. 2023. PMID: 37761971 Free PMC article. Review.
-
Astrocytes phenomics as new druggable targets in healthy aging and Alzheimer's disease progression.Front Cell Neurosci. 2025 Jan 6;18:1512985. doi: 10.3389/fncel.2024.1512985. eCollection 2024. Front Cell Neurosci. 2025. PMID: 39835288 Free PMC article. Review.
-
Different Patterns of Neurodegeneration and Glia Activation in CA1 and CA3 Hippocampal Regions of TgCRND8 Mice.Front Aging Neurosci. 2018 Nov 13;10:372. doi: 10.3389/fnagi.2018.00372. eCollection 2018. Front Aging Neurosci. 2018. PMID: 30483118 Free PMC article.
-
The neuron-astrocyte-microglia triad involvement in neuroinflammaging mechanisms in the CA3 hippocampus of memory-impaired aged rats.Exp Gerontol. 2016 Oct;83:71-88. doi: 10.1016/j.exger.2016.07.011. Epub 2016 Jul 25. Exp Gerontol. 2016. PMID: 27466072
Cited by
-
The effects and potential of microglial polarization and crosstalk with other cells of the central nervous system in the treatment of Alzheimer's disease.Neural Regen Res. 2023 May;18(5):947-954. doi: 10.4103/1673-5374.355747. Neural Regen Res. 2023. PMID: 36254973 Free PMC article. Review.
-
Nicotine suppresses crystalline silica-induced astrocyte activation and neuronal death by inhibiting NF-κB in the mouse hippocampus.CNS Neurosci Ther. 2024 Apr;30(4):e14508. doi: 10.1111/cns.14508. Epub 2023 Oct 21. CNS Neurosci Ther. 2024. PMID: 37864452 Free PMC article.
-
Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats dependent on NLRP3 activation.Metab Brain Dis. 2024 Nov 13;40(1):1. doi: 10.1007/s11011-024-01425-5. Metab Brain Dis. 2024. PMID: 39535569
-
Roles of neuropathology-associated reactive astrocytes: a systematic review.Acta Neuropathol Commun. 2023 Mar 13;11(1):42. doi: 10.1186/s40478-023-01526-9. Acta Neuropathol Commun. 2023. PMID: 36915214 Free PMC article.
-
Conditional Knockout of IL-1R1 in Endothelial Cells Attenuates Seizures and Neurodegeneration via Inhibiting Neuroinflammation Mediated by Nrf2/HO-1/NLRP3 Signaling in Status Epilepticus Model.Mol Neurobiol. 2024 Jul;61(7):4289-4303. doi: 10.1007/s12035-023-03842-6. Epub 2023 Dec 12. Mol Neurobiol. 2024. PMID: 38087170 Free PMC article.
References
-
- Bartsch T., Dähring J., Reuter S., Finke C., Rohr A., Brauer H., et al. . (2015). Selective neuronal vulnerability of human hippocampal CA1 neurons: lesion evolution, temporal course, and pattern of hippocampal damage in diffusion-weighted MR imaging. J. Cereb. Blood Flow Metab. 35, 1836–1845. 10.1038/jcbfm.2015.137 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

