GILZ Regulates the Expression of Pro-Inflammatory Cytokines and Protects Against End-Organ Damage in a Model of Lupus
- PMID: 33889157
- PMCID: PMC8056982
- DOI: 10.3389/fimmu.2021.652800
GILZ Regulates the Expression of Pro-Inflammatory Cytokines and Protects Against End-Organ Damage in a Model of Lupus
Abstract
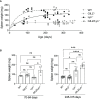
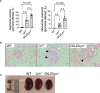
Glucocorticoid-induced leucine zipper (GILZ) mimics many of the anti-inflammatory effects of glucocorticoids, suggesting it as a point of therapeutic intervention that could bypass GC adverse effects. We previously reported that GILZ down-regulation is a feature of human SLE, and loss of GILZ permits the development of autoantibodies and lupus-like autoimmunity in mice. To further query the contribution of GILZ to protection against autoimmune inflammation, we studied the development of the lupus phenotype in Lyn-deficient (Lyn-/-) mice in which GILZ expression was genetically ablated. In Lyn-/- mice, splenomegaly, glomerulonephritis, anti-dsDNA antibody titres and cytokine expression were exacerbated by GILZ deficiency, while other autoantibody titres and glomerular immune complex deposition were unaffected. Likewise, in patients with SLE, GILZ was inversely correlated with IL23A, and in SLE patients not taking glucocorticoids, GILZ was also inversely correlated with BAFF and IL18. This suggests that at the onset of autoimmunity, GILZ protects against tissue injury by modulating pro-inflammatory pathways, downstream of antibodies, to regulate the cycle of inflammation in SLE.
Keywords: GILZ; IL-23; glomerulonephritis; glucocorticoid; systemic lupus erythematosus.
Copyright © 2021 Nataraja, Dankers, Flynn, Lee, Zhu, Vincent, Gearing, Ooi, Pervin, Cristofaro, Sherlock, Hasnat, Harris, Morand and Jones.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

