Carcinoma cells that have undergone an epithelial-mesenchymal transition differentiate into endothelial cells and contribute to tumor growth
- PMID: 33889304
- PMCID: PMC8057273
- DOI: 10.18632/oncotarget.27940
Carcinoma cells that have undergone an epithelial-mesenchymal transition differentiate into endothelial cells and contribute to tumor growth
Abstract
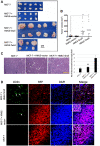
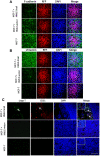
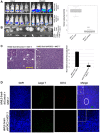
Hypoxia stimulates neoangiogenesis, promoting tumor outgrowth, and triggers the epithelial-mesenchymal transition (EMT), which bestows cells with mesenchymal traits and multi-lineage differentiation potential. Here, we investigated whether EMT can confer endothelial attributes upon carcinoma cells, augmenting tumor growth and vascularization. Following orthotopic implantation of MCF-7 human epithelial breast cancer cells into mice, tumors of different sizes were immunostained for markers of hypoxia and EMT. Larger tumors were well-vascularized with CD31-positive cells of human origin. Hypoxic regions, demarcated by HIF-1α staining, exhibited focal areas of E-cadherin loss and elevated levels of vimentin and the EMT-mediator FOXC2. Implantation of MCF-7 cells, co-mixed with human mammary epithelial (HMLE) cells overexpressing the EMT-inducer Snail, markedly potentiated tumor growth and vascularization, compared with MCF-7 cells injected alone or co-mixed with HMLE-vector cells. Intra-tumoral vessels contained CD31-positive cells derived from either donor cell type. FOXC2 knockdown abrogated the potentiating effects of HMLE-Snail cells on MCF-7 tumor growth and vascularization, and compromised endothelial transdifferentiation of mesenchymal cells cultured in endothelial growth medium. Hence, cells that have undergone EMT can promote tumor growth and neovascularization either indirectly, by promoting endothelial transdifferentiation of carcinoma cells, or directly, by acquiring an endothelial phenotype, with FOXC2 playing key roles in these processes.
Keywords: FOXC2; angiogenesis; endothelial transdifferentiation; epithelial-mesenchymal transition; vasculogenic mimicry.
Copyright: © 2021 Sphyris et al.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no conflicts of interest to declare.
Figures




Similar articles
-
Hypoxia-inducible factor 1α-induced epithelial-mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis.Hum Reprod. 2016 Jun;31(6):1327-38. doi: 10.1093/humrep/dew081. Epub 2016 Apr 19. Hum Reprod. 2016. PMID: 27094478
-
Hypoxia promotes vasculogenic mimicry formation by vascular endothelial growth factor A mediating epithelial-mesenchymal transition in salivary adenoid cystic carcinoma.Cell Prolif. 2019 May;52(3):e12600. doi: 10.1111/cpr.12600. Epub 2019 Apr 3. Cell Prolif. 2019. PMID: 30945361 Free PMC article.
-
Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma.Gynecol Oncol. 2014 Jun;133(3):575-83. doi: 10.1016/j.ygyno.2014.02.034. Epub 2014 Feb 28. Gynecol Oncol. 2014. PMID: 24589413
-
HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment.J Exp Clin Cancer Res. 2017 Apr 27;36(1):60. doi: 10.1186/s13046-017-0533-1. J Exp Clin Cancer Res. 2017. PMID: 28449718 Free PMC article.
-
Aneuploid Circulating Tumor-Derived Endothelial Cell (CTEC): A Novel Versatile Player in Tumor Neovascularization and Cancer Metastasis.Cells. 2020 Jun 24;9(6):1539. doi: 10.3390/cells9061539. Cells. 2020. PMID: 32599893 Free PMC article. Review.
Cited by
-
The Metabolic Landscape of Breast Cancer and Its Therapeutic Implications.Mol Diagn Ther. 2023 May;27(3):349-369. doi: 10.1007/s40291-023-00645-2. Epub 2023 Mar 29. Mol Diagn Ther. 2023. PMID: 36991275 Review.
-
Mechanisms of Cancer Cell Lymphatic Endothelialization in Tumor Lymphangiogenesis and Metastasis: A Comprehensive Review.J Inflamm Res. 2025 Aug 9;18:10741-10750. doi: 10.2147/JIR.S535351. eCollection 2025. J Inflamm Res. 2025. PMID: 40809467 Free PMC article. Review.
-
FOXC2 Promotes Vasculogenic Mimicry in Ovarian Cancer.Cancers (Basel). 2022 Oct 4;14(19):4851. doi: 10.3390/cancers14194851. Cancers (Basel). 2022. PMID: 36230774 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

