Combination immunotherapy with ipilimumab and nivolumab in patients with advanced adrenocortical carcinoma: a subgroup analysis of CA209-538
- PMID: 33889439
- PMCID: PMC8043165
- DOI: 10.1080/2162402X.2021.1908771
Combination immunotherapy with ipilimumab and nivolumab in patients with advanced adrenocortical carcinoma: a subgroup analysis of CA209-538
Abstract
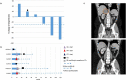
Background: Adrenocortical carcinoma is a rare malignancy, with poor prognosis and limited treatment options for patients with advanced disease. Chemotherapy is the current standard first-line treatment, providing only a modest survival benefit. There is only limited treatment experience with immunotherapy using single-agent anti-PD-1/PD-L1 therapy. To date no clinical trials have been reported using combination immunotherapy with anti-CTLA-4 and anti-PD-1 blockade in this patient population. Methods: CA209-538 is a prospective multicentre clinical trial in patients with advanced rare cancers. Participants received the anti-PD-1 antibody nivolumab (3 mg/kg IV) and the anti-CTLA-4 antibody ipilimumab (1 mg/kg IV) every three weeks for four doses, followed by nivolumab (3 mg/kg IV) every two weeks and continued for up to 96 weeks, until disease progression or unacceptable toxicity. Response was assessed every 12 weeks by RECIST version 1.1. Primary endpoint was clinical benefit rate (complete response, partial response, stable disease at 12 weeks). Results: Six patients with adrenocortical carcinoma were enrolled and received treatment. Two patients (33%) have an ongoing partial response (10 and 25 months +) and two patients (33%) stable disease leading to a disease control rate of 66%. Both responders had tumors with a microsatellite instable phenotype. One patient rapidly progressed shortly after enrollment into the trial and did not undergo restaging. Immunotherapy-related toxicity was reported in all patients, with four patients (67%) experiencing grade 3/4 hepatitis leading to discontinuation of treatment. Conclusions: This is the first treatment experience using ipilimumab and nivolumab combination immunotherapy in patients with advanced adrenocortical carcinoma. Durable responses have been observed in a subset of patients suggesting that this treatment regimen should be further investigated in this patient population.
Keywords: Adrenocortical carcinoma; anti-ctla-4; anti-pd-1; anti-pd-l1; ipilimumab; nivolumab.
© 2021 The Author(s). Published with license by Taylor & Francis Group, LLC.
Figures
References
-
- Fassnacht M, Assie G, Baudin E, et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1476–1490. [published Online First: 2020/08/31]. doi:10.1016/j.annonc.2020.08.2099. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
