Predicting Recurrent Venous Thromboembolism in Patients With Deep-Vein Thrombosis: Development and Internal Validation of a Potential New Prediction Model (Continu-8)
- PMID: 33889600
- PMCID: PMC8055939
- DOI: 10.3389/fcvm.2021.655226
Predicting Recurrent Venous Thromboembolism in Patients With Deep-Vein Thrombosis: Development and Internal Validation of a Potential New Prediction Model (Continu-8)
Abstract
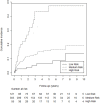
Background: Previous prediction models for recurrent thromboembolism (VTE) are often complicated to apply and have not been implemented widely. Aim: To develop and internally validate a potential new prediction model for recurrent VTE that can be used without stopping anticoagulant treatment for D-dimer measurements in patients with provoked and unprovoked DVT. Methods: Cohort data of 479 patients treated in a clinical care pathway at Maastricht University Medical Center were used. Predictors for the Cox proportional hazards model (unprovoked DVT, male gender, factor VIII levels) were derived from literature and using forward selection procedure. The scoring rule was internally validated using bootstrapping techniques and the predictive ability was compared to existing prediction models. Results: Patients were followed for a median of 3.12 years after stopping anticoagulation treatment (IQR 0.78, 3.90). Sixty-four of 479 patients developed recurrent VTE (13%). The scoring rule consisted of unprovoked DVT (yes: 2 points), male sex (yes: 1 point), and factor VIII > 213 % (yes: 2 points) and was categorized into three groups [i.e., low risk (score 0), medium risk (scores 1, 2, or 3) and high risk (scores 4 and 5)]. The concordance statistic was 0.68 (95% CI: 0.61, 0.75). Conclusion: The discriminative ability of the new Continu-8 score was adequate. Future studies shall verify this score in an independent setting without stopping anticoagulation treatment.
Keywords: clinical decision making; health services research; risk factors; venous thrombosis/epidemiology; venous thrombosis/mortality; venous thrombosis/therapy.
Copyright © 2021 Nagler, Van Kuijk, Ten Cate, Prins and Ten Cate-Hoek.
Conflict of interest statement
MN reports receiving grants from the Swiss National Science Foundation (SNSF), during the conduct of the study; and research grants from Bayer, Stago, Roche diagnostics, outside of the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
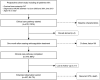
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

