Peginterferon and Entecavir Combination Therapy Improves Outcome of Non-Early Response Hepatitis B e Antigen-Positive Patients
- PMID: 33889654
- PMCID: PMC8050793
- DOI: 10.1093/ofid/ofaa462
Peginterferon and Entecavir Combination Therapy Improves Outcome of Non-Early Response Hepatitis B e Antigen-Positive Patients
Erratum in
-
Erratum to: Peginterferon and Entecavir Combination Therapy Improves Outcome of Non-Early Response Hepatitis B e Antigen-Positive Patients.Open Forum Infect Dis. 2021 Dec 21;8(12):ofab354. doi: 10.1093/ofid/ofab354. eCollection 2021 Dec. Open Forum Infect Dis. 2021. PMID: 34950746 Free PMC article.
Abstract
Background: The efficacy of nucleot(s)ide analogs (NAs) and pegylated interferon (PegIFN) combination therapy for hepatitis B e antigen-positive (HBeAg+) patients is still controversial. Whether PegIFN and entecavir (ETV) combination therapy could provide a greater benefit for HBeAg+ patients was assessed.
Methods: Treatment-naïve HBeAg+ patients initiated on PegIFN alfa-2a (PegIFNα-2a) for 24 weeks without early response (early response: HBsAg <1500 IU/mL and hepatitis B virus [HBV] DNA <105 copies/mL) were recruited in the current study. Among total of 94 patients, 51 were continued on PegIFNα-2a monotherapy, and 43 were offered PegIFNα-2a and ETV combined therapy.
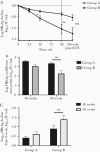
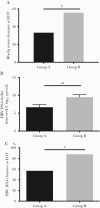
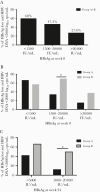
Results: Better outcomes in response to the combined therapy, compared with that of the monotherapy, were demonstrated, including more HBsAg decline and loss and HBV DNA decline and HBeAg clearance. Importantly, the patients with HBsAg levels between 1500 and 20 000 IU/mL initially or between 5000 and 20 000 IU/mL after 24 weeks of PegIFNα-2a benefitted more from the combined therapy, compared with those on monotherapy.
Conclusions: Combined therapy of PegIFNα-2a and ETV is more efficacious for HBeAg+ patients without early response to PegIFN monotherapy, and HBsAg levels are a good predictor of treatment outcomes.
Keywords: antiviral treatment; chronic hepatitis B; interferon; nucleos(t)ide analogues.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures

Similar articles
-
Effect of switching from treatment with nucleos(t)ide analogs to pegylated interferon α-2a on virological and serological responses in chronic hepatitis B patients.World J Gastroenterol. 2016 Dec 14;22(46):10210-10218. doi: 10.3748/wjg.v22.i46.10210. World J Gastroenterol. 2016. PMID: 28028369 Free PMC article. Clinical Trial.
-
Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial).J Hepatol. 2014 Oct;61(4):777-84. doi: 10.1016/j.jhep.2014.05.044. Epub 2014 Jun 7. J Hepatol. 2014. PMID: 24915612 Clinical Trial.
-
Phase IV randomized clinical study: Peginterferon alfa-2a with adefovir or entecavir pre-therapy for HBeAg-positive chronic hepatitis B.J Formos Med Assoc. 2018 Jul;117(7):588-597. doi: 10.1016/j.jfma.2017.12.007. Epub 2018 Feb 16. J Formos Med Assoc. 2018. PMID: 29456079 Clinical Trial.
-
Treatment of chronic hepatitis B virus infection - Dutch national guidelines.Neth J Med. 2008 Jul-Aug;66(7):292-306. Neth J Med. 2008. PMID: 18663260 Review.
-
Extended duration therapy regimens based on Pegylated interferon for chronic hepatitis B patients focusing on hepatitis B surface antigen loss: A systematic review and meta-analysis.Infect Genet Evol. 2020 Nov;85:104492. doi: 10.1016/j.meegid.2020.104492. Epub 2020 Aug 5. Infect Genet Evol. 2020. PMID: 32763441
References
-
- Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014; 384:2053–63. - PubMed
-
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–85. - PubMed
-
- Liaw YF, Kao JH, Piratvisuth T, et al. . Erratum to: Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int 2012; 6:809–10. - PubMed
LinkOut - more resources
Full Text Sources

