Scaffold-based developmental tissue engineering strategies for ectodermal organ regeneration
- PMID: 33889838
- PMCID: PMC8050778
- DOI: 10.1016/j.mtbio.2021.100107
Scaffold-based developmental tissue engineering strategies for ectodermal organ regeneration
Abstract
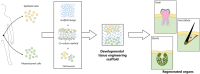
Tissue engineering (TE) is a multidisciplinary research field aiming at the regeneration, restoration, or replacement of damaged tissues and organs. Classical TE approaches combine scaffolds, cells and soluble factors to fabricate constructs mimicking the native tissue to be regenerated. However, to date, limited success in clinical translations has been achieved by classical TE approaches, because of the lack of satisfactory biomorphological and biofunctional features of the obtained constructs. Developmental TE has emerged as a novel TE paradigm to obtain tissues and organs with correct biomorphology and biofunctionality by mimicking the morphogenetic processes leading to the tissue/organ generation in the embryo. Ectodermal appendages, for instance, develop in vivo by sequential interactions between epithelium and mesenchyme, in a process known as secondary induction. A fine artificial replication of these complex interactions can potentially lead to the fabrication of the tissues/organs to be regenerated. Successful developmental TE applications have been reported, in vitro and in vivo, for ectodermal appendages such as teeth, hair follicles and glands. Developmental TE strategies require an accurate selection of cell sources, scaffolds and cell culture configurations to allow for the correct replication of the in vivo morphogenetic cues. Herein, we describe and discuss the emergence of this TE paradigm by reviewing the achievements obtained so far in developmental TE 3D scaffolds for teeth, hair follicles, and salivary and lacrimal glands, with particular focus on the selection of biomaterials and cell culture configurations.
Keywords: Cell coculture; Developmental; Epithelial-mesenchymal interaction; Gland regeneration; Hair follicle regeneration; Tooth regeneration; tissue engineering.
Crown Copyright © 2021 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

