Clinical and virological characteristics of hospitalised COVID-19 patients in a German tertiary care centre during the first wave of the SARS-CoV-2 pandemic: a prospective observational study
- PMID: 33890243
- PMCID: PMC8061715
- DOI: 10.1007/s15010-021-01594-w
Clinical and virological characteristics of hospitalised COVID-19 patients in a German tertiary care centre during the first wave of the SARS-CoV-2 pandemic: a prospective observational study
Abstract
Purpose: Adequate patient allocation is pivotal for optimal resource management in strained healthcare systems, and requires detailed knowledge of clinical and virological disease trajectories. The purpose of this work was to identify risk factors associated with need for invasive mechanical ventilation (IMV), to analyse viral kinetics in patients with and without IMV and to provide a comprehensive description of clinical course.
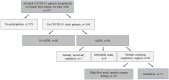
Methods: A cohort of 168 hospitalised adult COVID-19 patients enrolled in a prospective observational study at a large European tertiary care centre was analysed.
Results: Forty-four per cent (71/161) of patients required invasive mechanical ventilation (IMV). Shorter duration of symptoms before admission (aOR 1.22 per day less, 95% CI 1.10-1.37, p < 0.01) and history of hypertension (aOR 5.55, 95% CI 2.00-16.82, p < 0.01) were associated with need for IMV. Patients on IMV had higher maximal concentrations, slower decline rates, and longer shedding of SARS-CoV-2 than non-IMV patients (33 days, IQR 26-46.75, vs 18 days, IQR 16-46.75, respectively, p < 0.01). Median duration of hospitalisation was 9 days (IQR 6-15.5) for non-IMV and 49.5 days (IQR 36.8-82.5) for IMV patients.
Conclusions: Our results indicate a short duration of symptoms before admission as a risk factor for severe disease that merits further investigation and different viral load kinetics in severely affected patients. Median duration of hospitalisation of IMV patients was longer than described for acute respiratory distress syndrome unrelated to COVID-19.
Keywords: Artificial respiration; COVID-19 nucleic acid testing; Coronavirus disease 2019 (COVID-19); Mechanical ventilation; Prospective study; Respiratory distress syndrome; Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); Symptom assessment; Viral concentration.
© 2021. The Author(s).
Conflict of interest statement
Charlotte Thibeault, Barbara Mühlemann, Elisa T. Helbig, Mirja Mittermaier, Tilman Lingscheid, Pinkus Tober-Lau, Lil A. Meyer-Arndt, Leonie Meiners, Sascha S. Haenel, Laure Bosquillon de Jarcy, Lena Lippert, Moritz Pfeiffer, Miriam S. Stegemann, Robert Roehle, Janine Wiebach, Stefan Hippenstiel, Thomas Zoller, Holger Müller-Redetzky, Alexander Uhrig, Christof von Kalle, Norbert Suttorp, Terry C. Jones, Christian Drosten, Leif E. Sander, Victor M. Corman und Florian Kurth have no conflicts of interest to declare. CT reports grants within the frame of the academic program “clinical studies in infectiology” funded by the German Research Foundation (DFG), outside of the submitted work. MM reports grants from the BIH-Charité Digital Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin, the Berlin Institute of Health (BIH), and the German Research Foundation (DFG), outside the submitted work. FB reports grants from Einstein Foundation, personal fees from Axon Publishing, grants from Vifor Pharma, personal fees from Elsevier Publishing, grants from Federal Ministry of Health, grants from Berlin Institute of Health, grants from Federal Ministry of Education and Research, outside the submitted work.TCJ is in part funded through NIAID-NIH CEIRS contract HHSN272201400008C. MW reports grants from Deutsche Forschungsgemeinschaft, grants from Bundesministerium für Bildung und Forschung, grants from Deutsche Gesellschaft für Pneumologie, grants from European Respiratory Society, grants from Marie Curie Foundation, grants from Else Kröner Fresenius Stiftung, grants from Capnetz Stiftung, International Max Planck Research School, grants and personal fees from Actelion, grants and personal fees from Bayer Health Care, grants and personal fees from Biotest, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Noxxon, grants and personal fees from Pantherna, grants from Quark Pharma, grants and personal fees from Silence Therapeutics, grants from Takeda Pharma, grants and personal fees from Vaxxilon, personal fees from Aptarion, personal fees from Astra Zeneca, personal fees from Berlin Chemie, personal fees from Chiesi, personal fees from Glaxo Smith Kline, personal fees from Novartis, personal fees from Sinoxa, personal fees from Teva, outside the submitted work.
Figures


References
-
- Karagiannidis C, Mostert C, Hentschker C, Voshaar T, Malzahn J, Schillinger G, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. - DOI - PMC - PubMed
-
- Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

