Electrocatalytic Activation of Donor-Acceptor Cyclopropanes and Cyclobutanes: An Alternative C(sp3 )-C(sp3 ) Cleavage Mode
- PMID: 33890714
- PMCID: PMC8362004
- DOI: 10.1002/anie.202101477
Electrocatalytic Activation of Donor-Acceptor Cyclopropanes and Cyclobutanes: An Alternative C(sp3 )-C(sp3 ) Cleavage Mode
Abstract
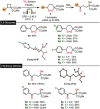
We describe the first electrochemical activation of D-A cyclopropanes and D-A cyclobutanes leading after C(sp3 )-C(sp3 ) cleavage to the formation of highly reactive radical cations. This concept is utilized to formally insert molecular oxygen after direct or DDQ-assisted anodic oxidation of the strained carbocycles, delivering β- and γ-hydroxy ketones and 1,2-dioxanes electrocatalytically. Furthermore, insights into the mechanism of the oxidative process, obtained experimentally and by additional quantum-chemical calculations are presented. The synthetic potential of the reaction products is demonstrated by diverse derivatizations.
Keywords: C(sp3)−C(sp3) cleavage; cyclobutanes; cyclopropanes; donor-acceptor systems; synthetic electrochemistry.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Friedel-Crafts-Type Reactions with Electrochemically Generated Electrophiles from Donor-Acceptor Cyclopropanes and -Butanes.Org Lett. 2021 Jul 16;23(14):5549-5553. doi: 10.1021/acs.orglett.1c01890. Epub 2021 Jul 7. Org Lett. 2021. PMID: 34231368
-
Organocatalytic enamine-activation of cyclopropanes for highly stereoselective formation of cyclobutanes.J Am Chem Soc. 2015 Feb 4;137(4):1685-91. doi: 10.1021/ja512573q. Epub 2015 Jan 21. J Am Chem Soc. 2015. PMID: 25575040
-
Synthesis of Functionalized Tetrahydropyridines by SnCl4 -Mediated [4+2] Cycloaddition between Donor-Acceptor Cyclobutanes and Nitriles.Chemistry. 2019 Dec 2;25(67):15244-15247. doi: 10.1002/chem.201903833. Epub 2019 Oct 31. Chemistry. 2019. PMID: 31529549
-
Ring contraction in synthesis of functionalized carbocycles.Chem Soc Rev. 2022 Oct 17;51(20):8652-8675. doi: 10.1039/d1cs01080h. Chem Soc Rev. 2022. PMID: 36172989 Review.
-
Donor-Acceptor Cyclopropanes in the Synthesis of Carbocycles.Chem Rec. 2019 Nov;19(11):2189-2208. doi: 10.1002/tcr.201800166. Epub 2019 Feb 1. Chem Rec. 2019. PMID: 30707497 Review.
Cited by
-
Reductive opening of a cyclopropane ring in the Ni(II) coordination environment: a route to functionalized dehydroalanine and cysteine derivatives.Beilstein J Org Chem. 2022 Sep 8;18:1166-1176. doi: 10.3762/bjoc.18.121. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 36128429 Free PMC article.
-
Electrochemical Deconstructive Methoxylation of Arylalcohols-A Synthetic and Mechanistic Investigation.Chemistry. 2024 Nov 21;30(65):e202403413. doi: 10.1002/chem.202403413. Epub 2024 Oct 30. Chemistry. 2024. PMID: 39287365 Free PMC article.
-
Strain-Release-Driven Electrochemical Skeletal Rearrangement of Non-Biased Alkyl Cyclopropanes/Butanes.Angew Chem Int Ed Engl. 2025 Jan 2;64(1):e202413723. doi: 10.1002/anie.202413723. Epub 2024 Oct 30. Angew Chem Int Ed Engl. 2025. PMID: 39264356 Free PMC article.
-
Donor-Acceptor Cyclopropanes: Activation Enabled by a Single, Vinylogous Acceptor.Angew Chem Int Ed Engl. 2023 Jan 2;62(1):e202214390. doi: 10.1002/anie.202214390. Epub 2022 Nov 30. Angew Chem Int Ed Engl. 2023. PMID: 36322458 Free PMC article.
-
Electroreductively Induced Radicals for Organic Synthesis.Molecules. 2023 Jan 15;28(2):857. doi: 10.3390/molecules28020857. Molecules. 2023. PMID: 36677915 Free PMC article. Review.
References
-
- Gordon M. S., J. Am. Chem. Soc. 1980, 102, 7419.
-
- None
-
- Reissig H.-U., Zimmer R., Chem. Rev. 2003, 103, 1151; - PubMed
-
- Novikov R. A., Potapov K. V., Chistikov D. N., Tarasova A. V., Grigoriev M. S., Timofeev V. P., Tomilov Y. V., Organometallics 2015, 34, 4238;
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

