VPS13D promotes peroxisome biogenesis
- PMID: 33891012
- PMCID: PMC8077185
- DOI: 10.1083/jcb.202001188
VPS13D promotes peroxisome biogenesis
Abstract
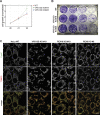
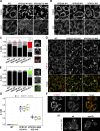
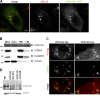
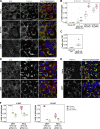
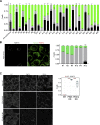
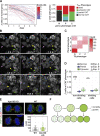
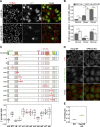
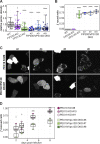
The VPS13 gene family consists of VPS13A-D in mammals. Although all four genes have been linked to human diseases, their cellular functions are poorly understood, particularly those of VPS13D. We generated and characterized knockouts of each VPS13 gene in HeLa cells. Among the individual knockouts, only VPS13D-KO cells exhibit abnormal mitochondrial morphology. Additionally, VPS13D loss leads to either partial or complete peroxisome loss in several transformed cell lines and in fibroblasts derived from a VPS13D mutation-carrying patient with recessive spinocerebellar ataxia. Our data show that VPS13D regulates peroxisome biogenesis.
© 2021 Baldwin et al.
Figures





References
-
- Abadi, M., Agarwal A., Barham P., Brevdo E., Chen Z., Citro C., Corrado G.S., Davis A., Dean J., Devin M., et al. 2016. TensorFlow: large-scale machine learning on heterogeneous distributed systems. http://tensorflow.org
-
- Anding, A.L., Wang C., Chang T.K., Sliter D.A., Powers C.M., Hofmann K., Youle R.J., and Baehrecke E.H.. 2018. Vps13D encodes a ubiquitin-binding protein that is required for the regulation of mitochondrial size and clearance. Curr. Biol. 28:287–295.e6. 10.1016/j.cub.2017.11.064 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

