The Panoramic ECAP Method: Estimating Patient-Specific Patterns of Current Spread and Neural Health in Cochlear Implant Users
- PMID: 33891218
- PMCID: PMC8476702
- DOI: 10.1007/s10162-021-00795-2
The Panoramic ECAP Method: Estimating Patient-Specific Patterns of Current Spread and Neural Health in Cochlear Implant Users
Abstract
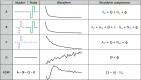
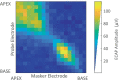
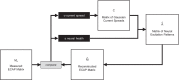
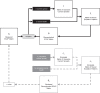
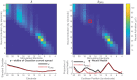
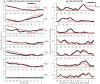
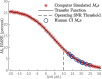
The knowledge of patient-specific neural excitation patterns from cochlear implants (CIs) can provide important information for optimizing efficacy and improving speech perception outcomes. The Panoramic ECAP ('PECAP') method (Cosentino et al. 2015) uses forward-masked electrically evoked compound action-potentials (ECAPs) to estimate neural activation patterns of CI stimulation. The algorithm requires ECAPs be measured for all combinations of probe and masker electrodes, exploiting the fact that ECAP amplitudes reflect the overlapping excitatory areas of both probes and maskers. Here we present an improved version of the PECAP algorithm that imposes biologically realistic constraints on the solution, that, unlike the previous version, produces detailed estimates of neural activation patterns by modelling current spread and neural health along the intracochlear electrode array and is capable of identifying multiple regions of poor neural health. The algorithm was evaluated for reliability and accuracy in three ways: (1) computer-simulated current-spread and neural-health scenarios, (2) comparisons to psychophysical correlates of neural health and electrode-modiolus distances in human CI users, and (3) detection of simulated neural 'dead' regions (using forward masking) in human CI users. The PECAP algorithm reliably estimated the computer-simulated scenarios. A moderate but significant negative correlation between focused thresholds and the algorithm's neural-health estimates was found, consistent with previous literature. It also correctly identified simulated 'dead' regions in all seven CI users evaluated. The revised PECAP algorithm provides an estimate of neural excitation patterns in CIs that could be used to inform and optimize CI stimulation strategies for individual patients in clinical settings.
Keywords: cochlear implant (CI); current spread; electrically evoked compound action potential (ECAP); neural excitation patterns; neural health; optimization.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

