Human motor units in microfluidic devices are impaired by FUS mutations and improved by HDAC6 inhibition
- PMID: 33891869
- PMCID: PMC8452598
- DOI: 10.1016/j.stemcr.2021.03.029
Human motor units in microfluidic devices are impaired by FUS mutations and improved by HDAC6 inhibition
Abstract
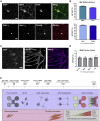
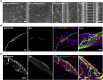
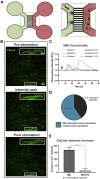
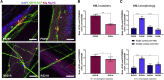
Neuromuscular junctions (NMJs) ensure communication between motor neurons (MNs) and muscle; however, in MN disorders, such as amyotrophic lateral sclerosis (ALS), NMJs degenerate resulting in muscle atrophy. The aim of this study was to establish a versatile and reproducible in vitro model of a human motor unit to investigate the effects of ALS-causing mutations. Therefore, we generated a co-culture of human induced pluripotent stem cell (iPSC)-derived MNs and human primary mesoangioblast-derived myotubes in microfluidic devices. A chemotactic and volumetric gradient facilitated the growth of MN neurites through microgrooves resulting in the interaction with myotubes and the formation of NMJs. We observed that ALS-causing FUS mutations resulted in reduced neurite outgrowth as well as an impaired neurite regrowth upon axotomy. NMJ numbers were likewise reduced in the FUS-ALS model. Interestingly, the selective HDAC6 inhibitor, Tubastatin A, improved the neurite outgrowth, regrowth, and NMJ morphology, prompting HDAC6 inhibition as a potential therapeutic strategy for ALS.
Keywords: FUS; HDAC6; Tubastatin A; amyotrophic lateral sclerosis; microfluidic device; neurite outgrowth; neurite regrowth; neuromuscular junction.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
L.V.D.B. has a patent on the use of HDAC inhibitors in Charcot-Marie-Tooth disease (US-2013227717-A1), is scientific co-founder of Augustine Therapeutics and a member of its scientific advisory board. The other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

