Sensitivity of SARS-CoV-2 RNA polymerase chain reaction using a clinical and radiological reference standard
- PMID: 33892014
- PMCID: PMC8057690
- DOI: 10.1016/j.jinf.2021.04.012
Sensitivity of SARS-CoV-2 RNA polymerase chain reaction using a clinical and radiological reference standard
Abstract
Objectives: Diagnostic tests for SARS-CoV-2 are important for epidemiology, clinical management, and infection control. Limitations of oro-nasopharyngeal real-time PCR sensitivity have been described based on comparisons of single tests with repeated sampling. We assessed SARS-CoV-2 PCR clinical sensitivity using a clinical and radiological reference standard.
Methods: Between March-May 2020, 2060 patients underwent thoracic imaging and SARS-CoV-2 PCR testing. Imaging was independently double- or triple-reported (if discordance) by blinded radiologists according to radiological criteria for COVID-19. We excluded asymptomatic patients and those with alternative diagnoses that could explain imaging findings. Associations with PCR-positivity were assessed with binomial logistic regression.
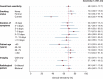
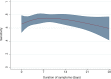
Results: 901 patients had possible/probable imaging features and clinical symptoms of COVID-19 and 429 patients met the clinical and radiological reference case definition. SARS-CoV-2 PCR sensitivity was 68% (95% confidence interval 64-73), was highest 7-8 days after symptom onset (78% (68-88)) and was lower among current smokers (adjusted odds ratio 0.23 (0.12-0.42) p < 0.001).
Conclusions: In patients with clinical and imaging features of COVID-19, PCR test sensitivity was 68%, and was lower among smokers; a finding that could explain observations of lower disease incidence and that warrants further validation. PCR tests should be interpreted considering imaging, symptom duration and smoking status.
Keywords: COVID-19; Diagnostic X-Ray; Diagnostic testing; Radiology; Real-time polymerase chain reaction; SARS-CoV-2; Sensitivity and specificity.
Copyright © 2021 The British Infection Association. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures
Similar articles
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Sep 30;9:CD013639. doi: 10.1002/14651858.CD013639.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. PMID: 32997361 Updated.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2020 Nov 26;11:CD013639. doi: 10.1002/14651858.CD013639.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Mar 16;3:CD013639. doi: 10.1002/14651858.CD013639.pub4. PMID: 33242342 Updated.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
-
SARS-CoV-2 serology increases diagnostic accuracy in CT-suspected, PCR-negative COVID-19 patients during pandemic.Respir Res. 2021 Apr 23;22(1):119. doi: 10.1186/s12931-021-01717-9. Respir Res. 2021. PMID: 33892720 Free PMC article.
-
Evaluation of performance of two SARS-CoV-2 Rapid IgM-IgG combined antibody tests on capillary whole blood samples from the fingertip.PLoS One. 2020 Sep 17;15(9):e0237694. doi: 10.1371/journal.pone.0237694. eCollection 2020. PLoS One. 2020. PMID: 32941461 Free PMC article.
Cited by
-
Swab and Sputum SARS-CoV-2 RNA-Negative, CT-Positive, Symptomatic Contacts of COVID-19 Cases: A Hypothesis-Generating Prospective Population-Based Cohort Study of Eight Clusters.Front Med (Lausanne). 2021 Aug 17;8:685544. doi: 10.3389/fmed.2021.685544. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34485329 Free PMC article.
-
When to test for COVID-19 using real-time reverse transcriptase polymerase chain reaction: a systematic review.Int J Infect Dis. 2022 Oct;123:58-69. doi: 10.1016/j.ijid.2022.06.037. Epub 2022 Jun 26. Int J Infect Dis. 2022. PMID: 35760382 Free PMC article.
-
The Diagnostic Performance of Various Clinical Specimens for the Detection of COVID-19: A Meta-Analysis of RT-PCR Studies.Diagnostics (Basel). 2023 Sep 26;13(19):3057. doi: 10.3390/diagnostics13193057. Diagnostics (Basel). 2023. PMID: 37835801 Free PMC article. Review.
-
Current tobacco use and COVID-19 diagnoses in a cohort of adult clients of public dental clinics in Sweden.Sci Rep. 2023 Jan 21;13(1):1204. doi: 10.1038/s41598-023-28091-4. Sci Rep. 2023. PMID: 36681700 Free PMC article.
References
-
- Venter M, Richter K. Towards effective diagnostic assays for COVID-19: a review. J Clin Pathol. 2020 PubMed PMID: 32404473. Epub 2020/05/15. eng. - PubMed
-
- Watson J, Whiting PF, Brush JE. Interpreting a covid-19 test result. BMJ. 2020;369:m1808. - PubMed
-
- Jarrom D, Elston L, Washington J, Prettyjohns M, Cann K, Myles S, et al. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: a rapid systematic review. BMJ Evid Based Med. 2020 PubMed PMID: 33004426. Epub 2020/10/03. eng. - PubMed
-
- Prokop M, van Everdingen W, van Rees Vellinga T, Quarles van Ufford H, Stöger L, Beenen L, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19-definition and evaluation. Radiology. 2020;296(2):E97–e104. Aug PubMed PMID: 32339082. Pubmed Central PMCID: PMC7233402. Epub 2020/04/28.eng. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

