miR-145 micelles mitigate atherosclerosis by modulating vascular smooth muscle cell phenotype
- PMID: 33892346
- PMCID: PMC8152375
- DOI: 10.1016/j.biomaterials.2021.120810
miR-145 micelles mitigate atherosclerosis by modulating vascular smooth muscle cell phenotype
Abstract
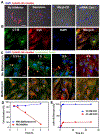
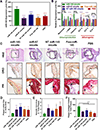
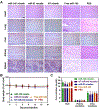
In atherosclerosis, resident vascular smooth muscle cells (VSMCs) in the blood vessels become highly plastic and undergo phenotypic switching from the quiescent, contractile phenotype to the migratory and proliferative, synthetic phenotype. Additionally, recent VSMC lineage-tracing mouse models of atherosclerosis have found that VSMCs transdifferentiate into macrophage-like and osteochondrogenic cells and make up to 70% of cells found in atherosclerotic plaques. Given VSMC phenotypic switching is regulated by microRNA-145 (miR-145), we hypothesized that nanoparticle-mediated delivery of miR-145 to VSMCs has the potential to mitigate atherosclerosis development by inhibiting plaque-propagating cell types derived from VSMCs. To test our hypothesis, we synthesized miR-145 micelles targeting the C-C chemokine receptor-2 (CCR2), which is highly expressed on synthetic VSMCs. When miR-145 micelles were incubated with human aortic VSMCs in vitro, >90% miR-145 micelles escaped the lysosomal pathway in 4 hours and released the miR cargo under cytosolic levels of glutathione, an endogenous reducing agent. As such, miR-145 micelles rescued atheroprotective contractile markers, myocardin, α-SMA, and calponin, in synthetic VSMCs in vitro. In early-stage atherosclerotic ApoE-/- mice, one dose of miR-145 micelles prevented lesion growth by 49% and sustained an increased level of miR-145 expression after 2 weeks post-treatment. Additionally, miR-145 micelles inhibited 35% and 43% plaque growth compared to free miR-145 and PBS, respectively, in mid-stage atherosclerotic ApoE-/- mice. Collectively, we present a novel therapeutic strategy and cell target for atherosclerosis, and present miR-145 micelles as a viable nanotherapeutic that can intervene atherosclerosis progression at both early and later stages of disease.
Keywords: Atherosclerosis; Micelle; Nanomedicine; Peptide; Smooth muscle cell; microRNA.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing interests
There are no competing interests to state.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
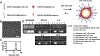


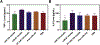


References
-
- Basatemur GL, et al. , Vascular smooth muscle cells in atherosclerosis. Nature Reviews Cardiology, 2019. 16(12): p. 727–744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

