GNA14 stimulation of KLF7 promotes malignant growth of endometrial cancer through upregulation of HAS2
- PMID: 33892667
- PMCID: PMC8066949
- DOI: 10.1186/s12885-021-08202-y
GNA14 stimulation of KLF7 promotes malignant growth of endometrial cancer through upregulation of HAS2
Abstract
Background: Endometrial cancer (UCEC) is one of the most common gynecological malignancies. We previously found that overexpression of G protein α subunit 14 (GNA14) promoted UCEC growth. Krüppel-like factor 7 (KLF7) acts as an oncogene in various cancer types, whereas the connection between GNA14 and KLF7 in UCEC is unclear. We herein explored the involvement of GNA14/KLF7 in UCEC development.
Methods: Clinical relevance of GNA14, KLF7 and HAS2 in UCEC was analyzed from TCGA and by immunohistochemical staining. Knockdown and overexpression of indicated genes were conducted by transfecting the cells with siRNAs and lentivirus, respectively. mRNA and protein expression was detected by qRT-PCR and Western blot. CCK8, colony formation, cell cycle, apoptosis, transwell and wound healing were performed to check cell biology function in vitro. Tumor growth in nude mice was conducted to check in vivo function. RNA sequencing was used to determine dys-regulated genes.
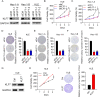
Results: We demonstrated that GNA14 stimulated the expression of KLF7 in UCEC cells. There was a positive correlation between GNA14 and KLF7 in normal and UCEC tissues. In vitro, KLF7 promoted cell proliferation, colony formation, cell cycle progression, and migration of UCEC cells. Apoptosis was inhibited by KLF7. Xenografted tumorigenesis of UCEC cells was suppressed by KLF7 knockdown. Furthermore, RNA sequencing results showed that KLF7 regulated the expression of a large amount of genes, among which hyaluronan synthase 2 (HAS2) was downregulated in KLF7 knockdown cells. Based on TCGA database and immunoblotting assays, KLF7 positively regulated HAS2 in UCEC cells and tissues. Lastly, knockdown of HAS2 reversed the oncogenic role of KLF7 on UCEC cell proliferation, migration, and xenografted tumor development.
Conclusion: Taken together, we reveal that GNA14/KLF7/HAS2 signaling cascade exerts tumor promoting function during UCEC development.
Keywords: Cancer development; Endometrial cancer; GNA14; HAS2; KLF7.
Conflict of interest statement
These authors declare no conflicts of interest.
Figures






References
-
- Braun M, Overbeek-Wager E, Grumbo R. Diagnosis and Management of Endometrial Cancer. Am Fam Physician. 2016;93(6):468–474. - PubMed
-
- Janku F, Wheler J, Westin S, Moulder S, Naing A, Tsimberidou A, Fu S, Falchook G, Hong D, Garrido-Laguna I, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol. 2012;30(8):777–782. doi: 10.1200/JCO.2011.36.1196. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

