The exosome journey: from biogenesis to uptake and intracellular signalling
- PMID: 33892745
- PMCID: PMC8063428
- DOI: 10.1186/s12964-021-00730-1
The exosome journey: from biogenesis to uptake and intracellular signalling
Abstract
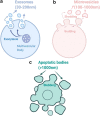
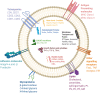
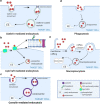
The use of exosomes in clinical settings is progressively becoming a reality, as clinical trials testing exosomes for diagnostic and therapeutic applications are generating remarkable interest from the scientific community and investors. Exosomes are small extracellular vesicles secreted by all cell types playing intercellular communication roles in health and disease by transferring cellular cargoes such as functional proteins, metabolites and nucleic acids to recipient cells. An in-depth understanding of exosome biology is therefore essential to ensure clinical development of exosome based investigational therapeutic products. Here we summarise the most up-to-date knowkedge about the complex biological journey of exosomes from biogenesis and secretion, transport and uptake to their intracellular signalling. We delineate the major pathways and molecular players that influence each step of exosome physiology, highlighting the routes of interest, which will be of benefit to exosome manipulation and engineering. We highlight the main controversies in the field of exosome research: their adequate definition, characterisation and biogenesis at plasma membrane. We also delineate the most common identified pitfalls affecting exosome research and development. Unravelling exosome physiology is key to their ultimate progression towards clinical applications. Video Abstract.
Keywords: Endocytosis; Exosomes; Extracellular vesicles; Intercellular communication; Lipid rafts; Multivesicular bodies; Rab GTPases; Targeting; Tetraspanins.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

