Remote monitoring technologies in Alzheimer's disease: design of the RADAR-AD study
- PMID: 33892789
- PMCID: PMC8063580
- DOI: 10.1186/s13195-021-00825-4
Remote monitoring technologies in Alzheimer's disease: design of the RADAR-AD study
Abstract
Background: Functional decline in Alzheimer's disease (AD) is typically measured using single-time point subjective rating scales, which rely on direct observation or (caregiver) recall. Remote monitoring technologies (RMTs), such as smartphone applications, wearables, and home-based sensors, can change these periodic subjective assessments to more frequent, or even continuous, objective monitoring. The aim of the RADAR-AD study is to assess the accuracy and validity of RMTs in measuring functional decline in a real-world environment across preclinical-to-moderate stages of AD compared to standard clinical rating scales.
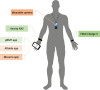
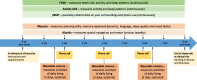
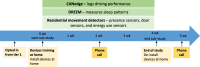
Methods: This study includes three tiers. For the main study, we will include participants (n = 220) with preclinical AD, prodromal AD, mild-to-moderate AD, and healthy controls, classified by MMSE and CDR score, from clinical sites equally distributed over 13 European countries. Participants will undergo extensive neuropsychological testing and physical examination. The RMT assessments, performed over an 8-week period, include walk tests, financial management tasks, an augmented reality game, two activity trackers, and two smartphone applications installed on the participants' phone. In the first sub-study, fixed sensors will be installed in the homes of a representative sub-sample of 40 participants. In the second sub-study, 10 participants will stay in a smart home for 1 week. The primary outcome of this study is the difference in functional domain profiles assessed using RMTs between the four study groups. The four participant groups will be compared for each RMT outcome measure separately. Each RMT outcome will be compared to a standard clinical test which measures the same functional or cognitive domain. Finally, multivariate prediction models will be developed. Data collection and privacy are important aspects of the project, which will be managed using the RADAR-base data platform running on specifically designed biomedical research computing infrastructure.
Results: First results are expected to be disseminated in 2022.
Conclusion: Our study is well placed to evaluate the clinical utility of RMT assessments. Leveraging modern-day technology may deliver new and improved methods for accurately monitoring functional decline in all stages of AD. It is greatly anticipated that these methods could lead to objective and real-life functional endpoints with increased sensitivity to pharmacological agent signal detection.
Keywords: Alzheimer’s disease; Remote monitoring technologies; Wearable technologies.
Conflict of interest statement
RK is an employee of Takeda Pharmaceuticals International Co. and holds stock options in both Pfizer Inc. and Takeda Pharmaceuticals International Co. IK receives consultancy fees from Mantrah Ltd. (mantrah.us), a digital technology company developing products for maintaining independence of people with cognitive impairment. CH is principal investigator on the Digital Biomarkers for Dementia project which is co-funded by Eli Lilly and Company and F. Hoffmann-La Roche Ltd. GE and KH are both currently employed by Novartis. NVM and VAN are employees of Janssen Pharmaceutica NV and may hold stock options or shares in the company. DA has received research support and/or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals, Biogen, and GE Health and served as a paid consultant for H. Lundbeck, Eisai, Heptares, and Mentis Cura.
Figures
References
-
- Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. - DOI - PMC - PubMed
-
- Amariglio RE, Donohue MC, Marshall GA, Rentz DM, Salmon DP, Ferris SH, Karantzoulis S, Aisen PS, Sperling RA, Alzheimer’s Disease Cooperative Study Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer’s Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. 2015;72(4):446–454. doi: 10.1001/jamaneurol.2014.3375. - DOI - PMC - PubMed
-
- Piau A, Wild K, Mattek N, Kaye J. Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild Alzheimer disease and implications for clinical care: systematic review. J Med Internet Res. 2019;21(8):e12785. doi: 10.2196/12785. - DOI - PMC - PubMed
-
- RADAR-AD. https://www.radar-ad.org/. Accessed 9 June 2020.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

