Integrative Analysis of Selected Metabolites and the Fungal Transcriptome during the Developmental Cycle of Ganoderma lucidum Strain G0119 Correlates Lignocellulose Degradation with Carbohydrate and Triterpenoid Metabolism
- PMID: 33893114
- PMCID: PMC8315967
- DOI: 10.1128/AEM.00533-21
Integrative Analysis of Selected Metabolites and the Fungal Transcriptome during the Developmental Cycle of Ganoderma lucidum Strain G0119 Correlates Lignocellulose Degradation with Carbohydrate and Triterpenoid Metabolism
Abstract
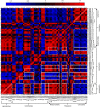
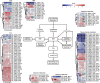
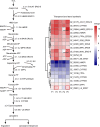
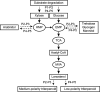
To systemically understand the biosynthetic pathways of bioactive substances, including triterpenoids and polysaccharides, in Ganoderma lucidum, the correlation between substrate degradation and carbohydrate and triterpenoid metabolism during growth was analyzed by combining changes in metabolite content and changes in related enzyme expression in G. lucidum over 5 growth phases. Changes in low-polarity triterpenoid content were correlated with changes in glucose and mannitol contents in fruiting bodies. Additionally, changes in medium-polarity triterpenoid content were correlated with changes in the lignocellulose content of the substrate and with the glucose, trehalose, and mannitol contents of fruiting bodies. Weighted gene coexpression network analysis (WGCNA) indicated that changes in trehalose and polyol contents were related to carbohydrate catabolism and polysaccharide synthesis. Changes in triterpenoid content were related to expression of the carbohydrate catabolic enzymes laccase, cellulase, hemicellulase, and polysaccharide synthase and to the expression of several cytochrome P450 monooxygenases (CYPs). It was concluded that the products of cellulose and hemicellulose degradation participate in polyol, trehalose, and polysaccharide synthesis during initial fruiting body formation. These carbohydrates accumulate in the early phase of fruiting body formation and are utilized when the fruiting bodies mature and a large number of spores are ejected. An increase in carbohydrate metabolism provides additional precursors for the synthesis of triterpenoids. IMPORTANCE Most studies of G. lucidum have focused on its medicinal function and on the mechanism of its activity, whereas the physiological metabolism and synthesis of bioactive substances during the growth of this species have been less studied. Therefore, theoretical guidance for cultivation methods to increase the production of bioactive compounds remains lacking. This study integrated changes in the lignocellulose, carbohydrate, and triterpenoid contents of G. lucidum with enzyme expression from transcriptomics data using WGCNA. The findings helped us better understand the connections between substrate utilization and the synthesis of polysaccharides and triterpenoids during the cultivation cycle of G. lucidum. The results of WGCNA suggest that the synthesis of triterpenoids can be enhanced not only through regulating the expression of enzymes in the triterpenoid pathway, but also through regulating carbohydrate metabolism and substrate degradation. This study provides a potential approach and identifies enzymes that can be targeted to regulate lignocellulose degradation and accelerate the accumulation of bioactive substances by regulating substrate degradation in G. lucidum.
Keywords: Ganoderma lucidum; carbohydrate; lignocellulose; triterpenoid.
Figures



Similar articles
-
Enhanced production of polysaccharides and triterpenoids in Ganoderma lucidum fruit bodies on induction with signal transduction during the fruiting stage.PLoS One. 2018 Apr 25;13(4):e0196287. doi: 10.1371/journal.pone.0196287. eCollection 2018. PLoS One. 2018. PMID: 29694432 Free PMC article.
-
Investigation of lignocellulolytic enzymes during different growth phases of Ganoderma lucidum strain G0119 using genomic, transcriptomic and secretomic analyses.PLoS One. 2018 May 31;13(5):e0198404. doi: 10.1371/journal.pone.0198404. eCollection 2018. PLoS One. 2018. PMID: 29852018 Free PMC article.
-
Triterpenes and Soluble Polysaccharide Changes in Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes), During Fruiting Growth.Int J Med Mushrooms. 2018;20(9):859-871. doi: 10.1615/IntJMedMushrooms.2018027357. Int J Med Mushrooms. 2018. PMID: 30317980
-
Strategies to Increase the Production of Triterpene Acids in Ligzhi or Reishi Medicinal Mushroom (Ganoderma lucidum, Agaricomycetes): A Review.Int J Med Mushrooms. 2024;26(5):25-41. doi: 10.1615/IntJMedMushrooms.2024052871. Int J Med Mushrooms. 2024. PMID: 38780421 Review.
-
Ganoderma lucidum and its pharmaceutically active compounds.Biotechnol Annu Rev. 2007;13:265-301. doi: 10.1016/S1387-2656(07)13010-6. Biotechnol Annu Rev. 2007. PMID: 17875480 Review.
Cited by
-
Gllac7 Is Induced by Agricultural and Forestry Residues and Exhibits Allelic Expression Bias in Ganoderma lucidum.Front Microbiol. 2022 Jun 30;13:890686. doi: 10.3389/fmicb.2022.890686. eCollection 2022. Front Microbiol. 2022. PMID: 35847055 Free PMC article.
-
Proteome study of dikaryotic and monokaryotic mycelia reveals the transfer between biological processes contributing to dikaryon growth advantage of the medicinal fungus Ganoderma lucidum.BMC Genomics. 2025 Aug 1;26(1):711. doi: 10.1186/s12864-025-11905-2. BMC Genomics. 2025. PMID: 40751175 Free PMC article.
-
Valorization of Green Biomass: Alfalfa Pulp as a Substrate for Oyster Mushroom Cultivation.Foods. 2022 Aug 20;11(16):2519. doi: 10.3390/foods11162519. Foods. 2022. PMID: 36010519 Free PMC article.
-
Integrated Transcriptomic and Targeted Metabolomic Analysis Reveals the Key Genes Involved in Triterpenoid Biosynthesis of Ganoderma lucidum.J Fungi (Basel). 2025 Jan 13;11(1):57. doi: 10.3390/jof11010057. J Fungi (Basel). 2025. PMID: 39852476 Free PMC article.
-
Comparative transcriptome analysis of genes and metabolic pathways involved in sporulation in Ganoderma lingzhi.G3 (Bethesda). 2022 Mar 4;12(3):jkab448. doi: 10.1093/g3journal/jkab448. G3 (Bethesda). 2022. PMID: 35079793 Free PMC article.
References
-
- Upton R, Graff A, Jolliffe G, Langer R, Williamson E. 2011. American herbal pharmacopoeia. CRC Press, Scotts Valley, CA.
-
- Xu S, Dou Y, Ye B, Wu Q, Wang Y, Hu M, Ma F, Rong X, Guo J. 2017. Ganoderma lucidum polysaccharides improve insulin sensitivity by regulating inflammatory cytokines and gut microbiota composition in mice. J Funct Foods 38:545–552. 10.1016/j.jff.2017.09.032. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

