Simultaneous Anaerobic and Aerobic Ammonia and Methane Oxidation under Oxygen Limitation Conditions
- PMID: 33893122
- PMCID: PMC8406961
- DOI: 10.1128/AEM.00043-21
Simultaneous Anaerobic and Aerobic Ammonia and Methane Oxidation under Oxygen Limitation Conditions
Abstract
Methane and ammonia have to be removed from wastewater treatment effluent in order to discharge it to receiving water bodies. A potential solution for this is a combination of simultaneous ammonia and methane oxidation by anaerobic ammonia oxidation (anammox) bacteria and nitrite/nitrate-dependent anaerobic methane oxidation (N-damo) microorganisms. When applied, these microorganisms will be exposed to oxygen, but little is known about the effect of a low concentration of oxygen on a culture containing these microorganisms. In this study, a stable coculture containing anammox and N-damo microorganisms in a laboratory scale bioreactor was established under oxygen limitation. Membrane inlet mass spectrometry (MIMS) was used to directly measure the in situ simultaneous activity of N-damo, anammox, and aerobic ammonia-oxidizing microorganisms. In addition, batch tests revealed that the bioreactor also harbored aerobic methanotrophs and anaerobic methanogens. Together with fluorescence in situ hybridization (FISH) analysis and metagenomics, these results indicate that the combination of N-damo and anammox activity under the continuous supply of limiting oxygen concentrations is feasible and can be implemented for the removal of methane and ammonia from anaerobic digester effluents. IMPORTANCE Nitrogen in wastewater leads to eutrophication of the receiving water bodies, and methane is a potent greenhouse gas; it is therefore important that these are removed from wastewater. A potential solution for the simultaneous removal of nitrogenous compounds and methane is the application of a combination of nitrite/nitrate-dependent methane oxidation (N-damo) and anaerobic ammonia oxidation (annamox). In order to do so, it is important to investigate the effect of oxygen on these two anaerobic processes. In this study, we investigate the effect of a continuous oxygen supply on the activity of an anaerobic methane- and ammonia-oxidizing coculture. The findings presented in this study are important for the potential application of these two microbial processes in wastewater treatment.
Keywords: ammonia oxidation; anaerobic methane oxidation; anammox; methane oxidation; nitrification; oxygen exposure; wastewater treatment.
Figures

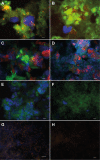



Similar articles
-
Diversity, enrichment, and genomic potential of anaerobic methane- and ammonium-oxidizing microorganisms from a brewery wastewater treatment plant.Appl Microbiol Biotechnol. 2020 Aug;104(16):7201-7212. doi: 10.1007/s00253-020-10748-z. Epub 2020 Jun 30. Appl Microbiol Biotechnol. 2020. PMID: 32607646 Free PMC article.
-
Interactions between anaerobic ammonium- and methane-oxidizing microorganisms in a laboratory-scale sequencing batch reactor.Appl Microbiol Biotechnol. 2019 Aug;103(16):6783-6795. doi: 10.1007/s00253-019-09976-9. Epub 2019 Jun 21. Appl Microbiol Biotechnol. 2019. PMID: 31227868 Free PMC article.
-
Coupling Partial Nitritation, Anammox and n-DAMO in a membrane aerated biofilm reactor for simultaneous dissolved methane and nitrogen removal.Water Res. 2024 May 15;255:121511. doi: 10.1016/j.watres.2024.121511. Epub 2024 Mar 24. Water Res. 2024. PMID: 38552483
-
Current perspectives on the application of N-damo and anammox in wastewater treatment.Curr Opin Biotechnol. 2018 Apr;50:222-227. doi: 10.1016/j.copbio.2018.01.031. Epub 2018 Feb 22. Curr Opin Biotechnol. 2018. PMID: 29477927 Review.
-
Enhancing mainstream nitrogen removal by employing nitrate/nitrite-dependent anaerobic methane oxidation processes.Crit Rev Biotechnol. 2019 Aug;39(5):732-745. doi: 10.1080/07388551.2019.1598333. Epub 2019 Apr 11. Crit Rev Biotechnol. 2019. PMID: 30971140 Review.
Cited by
-
Treatment of Anaerobic Digester Liquids via Membrane Biofilm Reactors: Simultaneous Aerobic Methanotrophy and Nitrogen Removal.Microorganisms. 2024 Sep 5;12(9):1841. doi: 10.3390/microorganisms12091841. Microorganisms. 2024. PMID: 39338515 Free PMC article.
References
-
- Myhre G, Shindell D, Pongratz J, et al. 2013. Anthropogenic and natural radiative forcing, p 659.–. InStocker TF, et al. (ed), Climate change 2013: the physical science basis. Contribution of Working Group I to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK.
-
- Ettwig KF, Butler MK, Le Paslier D, Pelletier E, Mangenot S, Kuypers MMM, Schreiber F, Dutilh BE, Zedelius J, de Beer D, Gloerich J, Wessels HJCT, van Alen TA, Luesken FA, Wu ML, van de Pas-Schoonen KT, Op den Camp HJM, Janssen-Megens EM, Francoijs KJ, Stunnenberg H, Weissenbach J, Jetten MSM, Strous M. 2010. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–549. 10.1038/nature08883. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

