Correlates of Neutralization against SARS-CoV-2 Variants of Concern by Early Pandemic Sera
- PMID: 33893169
- PMCID: PMC8223959
- DOI: 10.1128/JVI.00404-21
Correlates of Neutralization against SARS-CoV-2 Variants of Concern by Early Pandemic Sera
Abstract
Emerging SARS-CoV-2 variants of concern that overcome natural and vaccine-induced immunity threaten to exacerbate the COVID-19 pandemic. Increasing evidence suggests that neutralizing antibody (NAb) responses are a primary mechanism of protection against infection. However, little is known about the extent and mechanisms by which natural immunity acquired during the early COVID-19 pandemic confers cross-neutralization of emerging variants. In this study, we investigated cross-neutralization of the B.1.1.7 and B.1.351 SARS-CoV-2 variants in a well-characterized cohort of early pandemic convalescent subjects. We observed modestly decreased cross-neutralization of B.1.1.7 but a substantial 4.8-fold reduction in cross-neutralization of B.1.351. Correlates of cross-neutralization included receptor binding domain (RBD) and N-terminal domain (NTD) binding antibodies, homologous NAb titers, and membrane-directed T cell responses. These data shed light on the cross-neutralization of emerging variants by early pandemic convalescent immune responses. IMPORTANCE Widespread immunity to SARS-CoV-2 will be necessary to end the COVID-19 pandemic. NAb responses are a critical component of immunity that can be stimulated by natural infection as well as vaccines. However, SARS-CoV-2 variants are emerging that contain mutations in the spike gene that promote evasion from NAb responses. These variants may therefore delay control of the COVID-19 pandemic. We studied whether NAb responses from early COVID-19 convalescent patients are effective against the two SARS-CoV-2 variants, B.1.1.7 and B.1.351. We observed that the B.1.351 variant demonstrates significantly reduced susceptibility to early pandemic NAb responses. We additionally characterized virological, immunological, and clinical features that correlate with cross-neutralization. These studies increase our understanding of emerging SARS-CoV-2 variants.
Keywords: SARS-CoV-2; T cells; neutralizing antibodies.
Figures

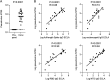


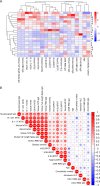
References
-
- Lumley SF, O'Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, Marsden BD, Cox S, James T, Warren F, Peck LJ, Ritter TG, de Toledo Z, Warren L, Axten D, Cornall RJ, Jones EY, Stuart DI, Screaton G, Ebner D, Hoosdally S, Chand M, Crook DW, O'Donnell AM, Conlon CP, Pouwels KB, Walker AS, Peto TEA, Hopkins S, Walker TM, Jeffery K, Eyre DW, Oxford University Hospitals Staff Testing Group . 2021. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 384:533–540. 10.1056/NEJMoa2034545. - DOI - PMC - PubMed
-
- Harvey RA, Rassen JA, Kabelac CA, Turenne W, Leonard S, Klesh R, Meyer WA, III, Kaufman HW, Anderson S, Cohen O, Petkov VI, Cronin KA, Van Dyke AL, Lowy DR, Sharpless NE, Penberthy LT. 2021. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med 10.1001/jamainternmed.2021.0366. - DOI - PMC - PubMed
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr., Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Group CCT, C4591001 Clinical Trial Group . 2020. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603–2615. 10.1056/NEJMoa2034577. - DOI - PMC - PubMed
-
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, COVE Study Group . 2021. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384:403–416. 10.1056/NEJMoa2035389. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

